Abstract
Purpose
The stomachs and spiral valves of sharks and rays were examined for their trypanorhynch (Cestoda) parasite fauna and dietary items to infer feeding ecology. In Indonesia, sharks and rays have been experiencing increasing awareness and conservation in the recent years due to high fisheries activities and to avoid future species extinction.
Methods
The samples were collected in 2009 from two different sampling sites at the southern coasts of Java and Bali in Indonesia. The parasite fauna was studied for 41 elasmobranch fishes. Amongst these, three shark species, Carcharhinus sorrah, Carcharhinus sp. I and Squalus megalops and seven ray species, Brevitrygon heterura, B. cf. heterura, Gymnura zonura, Maculabatis gerrardi, Mobula kuhlii, Neotrygon cauruleopuncatata and Rhinobatos penggali were studied. Four additional specimens, belonging to the shark species Carcharhinus sp. II and Mustelus cf. manazo and the ray species Maculabatis gerrardi were studied from the waters of South Bali.
Results
Analyses of the feeding ecology of the ray M. gerrardi revealed distinct differences between both sampling sites, indicating the presence of ecological differences between the geographically independent regions. A total of 11 different trypanorhynch species/taxa belonging to the five families Eutetrarhynchidae (5), Gilquiniidae (1), Lacistorhynchidae (1), Pterobothriidae (1) and Tentaculariidae (3) were found. Ten trypanorhynch species from Penyu Bay and four species from South Bali could be identified. Two taxa that might represent new species were collected: Dollfusiella sp. from Brevitrygon heterura and Prochristianella sp. from Maculabatis gerrardi.
Conclusions
The present paper gives insights in using the trypanorhynch cestode community in combination with feeding ecology analyses to support conservation of elasmobranchs in Indonesian waters.
Similar content being viewed by others
Introduction
With more than 200 species of sharks and rays, Indonesia is considered to have one of the richest elasmobranch faunas in the world. However, it is also a region where shark and ray populations are amongst the most heavily exploited with a reported 105,000 and 118,000 tonnes landed in 2002 and 2003, respectively [1,2,3]. Elasmobranchs are caught within Indonesian waters by both target fisheries and as bycatch by both small-scale fishers and commercial operators. A variety of fishing methods are used in the target fisheries, such as gill and tangle nets, longlines and harpoon [3]. Fisheries that land substantial catches of elasmobranchs as bycatch, include those operating bottom trawls, trammel and gill nets, longlines and droplines [1,2,3]. Available biological data or information concerning size compositions of species landed are scarce [3, 4]. The taxonomic and ecological knowledge of Indonesia’s elasmobranch fauna needs improving to provide an adequate baseline for data acquisition and resource management [3].
Ecology of Sampled Elasmobranchs
As apex predators, elasmobranchs play an important role in the marine food-web. Sharks and rays prey on a large range of organisms and therefore reductions in their population size can initiate trophic cascades through top–down effects [5,6,7]. Their diet includes crustaceans, cephalopods, bony fish (Teleostei) and other elasmobranch species (e.g., Squalus megalops; [18]). When considering their broad distribution and their extensive diet range, elasmobranchs are a crucial component in the energy transfer and food-web dynamics of the ocean [22,23,24].
The total world catch from wild marine stocks has increased in the recent years [8]. Fishing pressure and habitat loss have resulted in substantial declines in shark populations [5, 7, 9] resulting in approximately 40% of shark species being threatened with extinction [10]. Elasmobranchs show a high vulnerability to overfishing and other threats, such as habitat loss, due to their slow life cycle [11,12,13,14]. Nearly all of the examined shark and ray species within this study have, as do most elasmobranchs, an ovoviviparous reproduction (e.g., Squalus montalbani) where the embryos remain in the mother’s body until they are ready to hatch [15, 16]. Gestation periods up to 2 years (e.g., S. montalbani; [17]), sexual and geographical segregation of sexes (e.g., Scymnodon plunket; [12, 18, 19]) and late maturity (e.g., 22 years in the female Centroscymnus creptidater; [20]) are important factors that contribute to the relatively low reproductive rate of these cartilaginous fishes [21].
According to the IUCN Red List of Threatened Species [25], the conservation status of elasmobranch species that were examined in this study ranges from near threatened (Brevitrygon heterura) to vulnerable (Rhinobatos penggali) (Table 1). The government of Indonesia protect 11 species of elasmobranchs, i.e., whale shark (Rhincodon typus), manta ray (Mobula alfredi and M. birostris), freshwater rays (Fluvitrygon oxyrhincha, F. signifier and Urogymnus polylepis) and saw fish (Anoxypristis cuspidata and Pristis spp.). Additionally, they regulate the fishing of several species listed under CITES, including an export ban for thresher shark (Alopias spp.) and quotas for hammerhead sharks (Sphyrna spp.). Moreover, a few provincial governments have been developing initiatives to regulate fishing effort on sharks to control fishing mortality.
Elasmobranch Health
Questions concerning protection, conservation and the general health status of endangered elasmobranchs, have risen frequently in the recent years. As many elasmobranch species are endangered, their health status needs to be especially focussed on for future conservation (Table 1). Elasmobranchs are difficult to observe in nature and information on rare or less-frequently caught species is scarce [26]. This particularly concerns aspects of their ecology, main habitat, migration patterns, depth range and most important prey items [26] as well as health status and parasitism. According to Palm [27], fish parasites have been widely used as biological indicators for the host ecology, but the methods applied for the elasmobranchs are, so far, limited due to often restricted and unpredictable catches and less availability of specimens to study [26]. Parasitic diseases are increasingly recognized for their profound influences on individual, population and even ecosystem health [28]. Elasmobranchs have been reported as hosts of parasitic species, especially belonging to the order Trypanorhyncha Diesing, 1863, members of the Cestoda [26].
According to Palm [29], the Trypanorhyncha are distributed worldwide and amongst the most species-rich orders of marine cestodes. They infect stomachs and intestines of elasmobranchs as final hosts. Their larval stages occur in a wide range of organs of teleost fishes and a variety of marine invertebrates [29].
Study Areas
The city of Cilacap in Penyu Bay, South Central Java is surrounded by the brackish Segara Anakan lagoon, and is characterized by an oil refinery plant, a deep-sea harbour, a cement and a fertilizer factory. The lagoon plays an important role as a mating-, breeding- and nursery ground for a vast number of aquatic organisms. It is the last extensive mangrove system on Java [30]. The lagoon is impacted by sedimentation and deforestation [31], with pollution like heavy metals, pesticides, hydro carbonates and sediments [32] and minor to moderate nutrient pollution and eutrophication [33]. Although most coasts of the Indonesian archipelago have direct access to the deep-sea (below 200 m), the coastline of Penyu Bay, is comparatively shallow. According to Palm and Rückert [30, 34], the Segara Anakan lagoon has underlying inconsistent, varying environmental influences, including the change of rainy- and dry season, sedimentation and turbidity and differences in salinity. Fisheries constitute a main part of the local economy. The most important commercial fish species in the fish markets in Cilacap are tunas, sharks and rays. Inside the lagoon, many economically important species, such as milkfish and grouper, are farmed in aquaculture facilities.
The Bay of Kedonganan is located at the western side of the southern tip of Bali, directly next to the Ngurah Rai Kuta International Airport. The airstrip of the airport reaches into the ocean and acts as the northern border of the bay. There is no harbour; the small ships lay directly in front of the beach which is used to land captures. The fishermen catch fish from the Bali Strait and from areas close to South Bali and East Java. Kedonganan is a fishing village but is heavily influenced by tourism. A cooperation of the local fishermen manages the market [35]. In the fish market in Kedonganan, scombrids (tunas, mackerels and bonitos) as well as sharks are the most important species. Additional fishes imported from other Indonesian regions are also available.
The present study aimed to identify the trypanorhynch community of elasmobranchs from the southern Balinese and Javanese coasts. The relationship of the recorded parasite fauna with the elasmobranch feeding ecology is discussed, including the vulnerable (e.g., Brevitrygon heterura, Squalus megalops) to endangered (e.g., Gymnura zonura, Rhinobatos penggali) classified shark and ray species from Indonesian waters (Table 1).
Material and Methods
Sample Collection
The parasite fauna of 45 elasmobranch specimens was studied from the primary sampling site at Penyu Bay, South Central Java, in 2009 (Table 2, Table 3). Amongst these were the three shark species Carcharhinus sorrah (Carcharhinidae, Jordan & Evermann, 1896), Carcharhinus sp. I (Carcharhinidae), and Squalus megalops (Squalidae, Blainville, 1816), and the seven ray species Brevitrygon heterura (Dasyatidae, Jordan & Gilbert, 1879), B. cf. heterura (Dasyatidae), Gymnura zonura (Gymnuridae, Fowler, 1934), Maculabatis gerrardi (Dasyatidae) and Rhinobatos penggali (Rhinobatidae, Bonaparte, 1835), Mobula kuhlii (Myliobatidae, Bonaparte, 1835) and Neotrygon caeruleopunctata (Dasyatidae). The two shark species Carcharhinus sp. II (Carcharhinidae) and Mustelus cf. manazo (Triakidae, Gray, 1851) and the ray species M. gerrardi (Dasyatidae) were studied from the waters of South Bali in the same year.
Fish species were identified at the markets, TPI (Tempat Pelelangan Ikan) Teluk Penyu, Cilacap, southern Java coast (Penyu Bay) and Pasar Ikan Tradisional Kedonganan, southern Bali coast (Kuta Jimbaran Bay) with relevant literature (e.g., [3, 36, 37]. Morphometric data were taken through measuring the total length (TL, anterior tip to end of tail), standard length (to beginning of tail), body width (BW, for rays only) and, for sharks, pectoral fin and caudal fin length (PFL, CFL). Photos of the habitus and various characteristics necessary for identification were taken for subsequent taxonomy. Fish weights were taken at the market by means of the official market scales. Spiral valves (stomach and intestine) were isolated by means of knifes and scissors, and were then transported on ice to the local laboratories in the Biology Faculty of UNSOED (Java) and Veterinary Faculty of UDAYANA (Bali), where they were stored in freezers at −20 °C.
Parasitological Examination
Frozen intestinal tracts were thawed and weighed with and without contents. To release parasites from the spiral folds, the intestinal tract was cut into smaller pieces and shaken in NaCl 0.9% physiological solution in small bottles (200 ml) following the gut wash methodology of Cribb and Bray [38]. Liquids and tissues were then decanted in the dishes. Parasites were isolated from petri dishes, e.g., via further scraping off from tissue folds, under Zeiss Stemi DV4 and Olympus SZ2-ILST SZ51 binocular magnifiers.
Isolated parasites were cleaned from host tissue and gut contents and fixed in 70% EtOH, buffered with 4% formalin, and stored in 70% EtOH for subsequent analyses, e.g., microscopy. Cestoda were then stained with aceto-carmine (Mayer-Schuhberg’s, according to the protocol of Palm [29]) and mounted in Canada balsam. Drawings and photographs were prepared by means of a stereomicroscope Olympus CH-2, equipped with a Camera Lucida drawing tube (Leitz Wetzlar) and a BX50 microscope with a E410 camera (both Olympus). Scanning electron microscopy was performed with a LEO 1430 VP at the Heinrich-Heine University of Düsseldorf, after sputtering samples with gold–palladium in an argon atmosphere, according to the standard lab procedure, e.g., with 20.1 kV and different magnifications. The cestode species were identified according to Khalil et al. [39] and Palm [29].
Parasitological descriptors and infection rates, e.g., prevalence, (mean) abundance and (mean) intensity (P, mA, mI) were calculated according to standard procedures [40].
Stomach Content Analysis
The stomach contents were sorted, and prey items were identified to the lowest possible taxon and grouped into broad taxonomic categories (Teleostei, Cephalopoda, Crustacea, Euphausiacea, Decapoda, Gastropoda); we grouped (combined) Crustacea, Euphausiacea, and Decapoda into Crustacea for subsequent analysis. To determine the relative importance of prey items, the numerical percentage of prey (N%), the weight percentage of prey (W%) and the frequency of occurrence (F%) were determined [41, 42] combined over all individuals of a species. Using these three indices, the index of relative importance, IRI [43], was calculated. The importance of a specific prey item increases with higher values for N, W, F and IRI.
Statistics
Multi-variate statistical analyses were conducted with the Primer program (release 7, Primer-E Ltd. 7.0.13). Prior to the analyses, the parasite community data were fourth root-transformed to avoid an over-evaluation of rare or very frequent species. A similarity matrix was constructed using the Bray–Curtis similarity measure. Cluster analysis and non-metric multi-dimensional scaling (nMDS) were performed using the Primer software which was used to create two-dimensional ordination genera plot ([44, 45] then was clustered using group-average linking method (Field et al. [46])).
An one-way analyses of similarity were applied to determine the differences in community structure of parasite species composition between locations (routine ANOSIM, values close to 1 indicate high differences and close to 0 indicate high similarity between species compositions). Routine Similarity Percentage (SIMPER) analyses were applied to test which parasite species contributed most to show differences between stations. SIMPER analysis was used to determine which species was most responsible for the differences seen between sites with Bray–Curtis analysis (according to Bell and Barnes [47]).
Results
Fish Biological Data
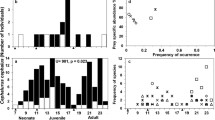
A total of 45 elasmobranch individuals were analysed within the present study (29 rays from seven species and 16 sharks from five species, Fig. 1).
Within the rays, Mobula kuhlii was the largest and heaviest species with a body length of 64.0 cm and a body weight of 12000 g. Within the sharks, Carcharhinus sorrah had the largest average size of 95.5 cm and the highest mean body weight of 4175 g (Table 2, Table 3).
Stomach Contents
In general, the differences in prey items in both rays and sharks were low (Table 4, Table 5). Diet of the studied rays consisted of Teleostei, Crustacea (combined) and Gastropoda, with Crustacea being the most abundant prey item. Brevitrygon heterura exclusively fed on crustaceans (IRI = 16,000 and 8000, respectively), while Maculabatis gerrardi and Gymnura zonura had the most varied diet consisting of Teleostei, Crustacea and Gastropoda. The studied sharks Carcharhinus sp. II exclusively fed on Teleostei (IRI = 20,000). Mustelus cf. manazo, Carcharhinus sorrah and Carcharhinus sp. I had the more diverse diet and preyed upon Teleostei, Cephalopoda and Crustacea.
Dietary composition, in the form of prey group found in each sample, was subjected to non-metric nMDS ordination in Fig. 2. Overall, several distinctive groups were formed which indicate different compositions of their diet. The genera Brevitrygon and Maculabatis (and, in part, Rhinobatos) formed the most evident cluster. The Brevitrygon and Maculabatis species group was characterized by Crustacea (dominated by Euphausiacea) as their main prey item. The remaining clusters of Carcharhinus, Mobula and Mustelus, were characterized by their more variable diet across Teleostei, Crustacea and Cephalopoda. Within this group, the smaller cluster of Carcharhinus, Rhinobatos and Squalus, had cephalopods as their main prey items (Fig. 2).
Parasite Infections
Ten different trypanorhynch species were identified from the elasmobranchs from Penyu Bay (PB), Cilacap, Java (Table 6). Most species have previously been recorded from Indonesian waters ([29]), however Nybelinia lingualis (Dollfus, 1929) is newly reported from this region and is described below. The single collected plerocercoid had the uncinate hooks with anterior extension of the base in the metabasal armature with the length of 13.67–14.6 μm and base length of 12.07–13.82 μm. In the basal armature, the basal hooks had the length of 8.15–10.59 μm and the base length of 8.22–10.5 μm.
Other characters were a tentacle width (TW) basal = 50 µm, TW metabasal = 43 µm and basal swelling absent. Tentacle sheath straight, Tentacle sheath width = 18. Half spiral row (hsr) = 7 basal, hsr = 7 metabasal. This character combination indicated conspecificity to this most commonly reported trypanorhynch of rays, especially from the Atlantic Ocean.
Two species, Dollfusiella sp. (Campbell & Beveridge, 1994) from Brevitrygon cf. heterura of Penyu Bay, Cilacap, Java and Prochristianella sp. (Dollfus, 1946) from M. gerrardi from Jimbaran, Kedonganan, Bali, were not identified to species level. Both had an unrecognized character combination, especially of the tentacular armature and might represent new species.
Within the families Gilquiniidae (Dollfus, 1935), Lacistorhynchidae (Guiart, 1937) and Pterobothriidae (Pintner, 1931) only one species was found respectively, while two species of Tentaculariidae (Poche, 1926) and four species of the family Eutetrarhynchidae (Guiart, 1927) were identified from Java. Rays and sharks from the Balinese sampling included four different parasite species (Table 7); three species belonged to the Eutetrarhynchidae and one species to the Tentaculariidae.
Parachristianella monomegacantha (Kruse, 1959) (from R. penggali) and P. baverstocki (Beveridge, 1990) (from M. gerrardi) showed the highest prevalence (100% each) and mean intensities (29.2 and 12.0), respectively, in rays from each collection location (Table 6, Table 7). Gilquinia robertsoni (Beveridge, 1990) (from S. megalops) showed the highest prevalence (60%) and mean intensity (16.7) in sharks collected from Java (Table 6). None of the sharks collected from Bali were infected with cestodes. For C. sorrah, Gy. zonura and Mustelus cf. manazo, no parasites were recorded. Analysis of multi-dimensional scaling provided information on similarities of parasites of host samples collected from Penyu Bay (Central Java) and Jimbaran (Bali). The analysis resulted in two major groups, which is likely to be formed based on host genus level. One group is formed by the host genera Brevitrygon, Maculabatis, and Rhinobatus, implying a closer relationship of the parasitological communities of these three genera than those of Squalus, which formed the other cluster. However, one group of the Maculabatis samples taken from Jimbaran formed a different cluster, implying dissimilarities for Maculabatis from Penyu Bay, due to the presence of the parasite species Kotorella pronosoma, (Euzet & Radujkovic, 1989) and genera Dollfusiella sp. and Prochristianella sp. The genera Brevitrygon, Maculabatis and Rhinobatus from Penyu Bay shared their similarity, especially in terms of Parachristianella baverstocki and P. monomegacantha and Parachristianella spp. The cluster of the genus Squalus is likely to be formed due to the presence of the cestode Gilquinia robertsoni and/or the absence of the genera Parachristinella (Fig. 3).
Interactions between Host Diet and Parasite Infections
The teleost dominated diet of the sharks Carcharhinus sp. 1 (100%) and Squalus megalops (95.2% Teleostei in stomach content in weight percent) is reflected in the parasite infection. Gilquinia robertsoni and Grillotia yuniariae (Palm, 2004) were detected in S. megalops and Mixonybelinia lepturi (Palm, 2004) in Carcharhinus sp. 1. The larval stages of Gr. yuniariae and Mi. lepturi are known from many teleost fishes [29].
Parachristianella monomegacantha, is amongst the most abundant and frequent parasite species within this study. Its transmission pathway and life cycle include Crustacea (Copepoda) as 1st intermediate hosts and Crustacea (shrimps, possibly euphausiids and crabs) as 2nd intermediate hosts before finally infecting rays (life cycle described in Mudry and Dailey [48]). It is also known that the life cycle for species of Dollfusiella, Parachristianella and Prochristianella include Crustacea as intermediate hosts [49,50,51]. Owing to the high infection rates observed, Crustacea must play an important role within the feeding ecology of the investigated hosts (Brevitrygon heterura (100%), Maculabatis gerrardi (100%), Neotrygon caeruleopunctata (100%), and Rhinobatos penggali (72.8% Crustacea in stomach content in weight percent)) of these trypanorhynch cestodes.
Discussion
The results of the present study help to fill gaps in general ecological knowledge, like feeding preferences, for several elasmobranch species from Indonesian waters. The results also combine the trypanorhynch fauna with reported dietary items to help determine the routes of transfer of trypanorhynch cestodes to these elasmobranchs. We are aware that this study, similar to Palm et al. [26], relied on opportunistic sampling of a number of elasmobranch species, with limited possibilities for data extrapolation. However, as any biological and ecological data about free-living sharks and rays is scarce, all available resources concerning feeding ecology and ecological preferences, health status and parasitism, must be considered.
The shark and ray species examined in the present study were mainly benthic species based on their stomach contents. In general, teleost fishes and cephalopods, including squid and octopus, were the major diet of the sharks, while the rays had diets dominated by crustaceans. The examined sharks and rays mostly had similar ranges of total lengths (less than 100 cm) and preyed upon the same benthic organisms in the same regions [52, 53]. Many of the elasmobranch species studied have limited zoogeographical distribution ranges, being endemic in Indonesia waters or specifically endemic to the southern waters of Java and Bali. On the other hand, Mobula kuhlii and Squalus megalops are usually rarely caught and landed in coastal waters. Squalus megalops is a deep-sea shark from the Central Indo-Pacific, while M. kuhlii is a pelagic ray species; both of these species were able to be included in this study due to the landing site, which was located on the edge of the Indian Ocean.
The studied parasite community and feeding ecology revealed certain patterns within the examined group of sharks and rays. The analysis of the feeding ecology showed several clusters with partially overlapping host groups. In particular, the trypanorhynch parasite community of S. megalops showed a distinct separation as compared to those of the other elasmobranchs, which clustered together. This clear separation can be explained by the ecology (see above), as well as other factors which may drive the differences. Squalus megalops occurs in depths up to 732 m [54], and it has a different range compared with the other sharks which are commonly known as coastal species. According to Palm et al. [26], influencing factors in addition to the diet may be depth orientation of the fishes, their habitat or their phylogeny/taxonomic classification. Palm et al. [26] stated in their study that the most remarkable relationship exists between the habitat type and the trypanorhynch assemblage. As Palm et al. [26] stated, transmission through the marine food web (via predation) and an unambiguous identification in the final (sharks and rays) and intermediate hosts (teleosts, other marine invertebrates) allow conclusions to be made on the feeding biology of the host. Their study demonstrated that nMDS of the elasmobranch parasitic Trypanorhyncha was a useful tool for investigating parasites as indicators of the host biology in marine ecosystems, especially depth distribution, diet, and habitat type were the major influencing factors. This seems to be the case also in the present study, where S. megalops clustered separately to the other sampled elasmobranch species.
Information on parasitic diseases, or other infections, and epidemiology and infection parameters are scarce on free-living elasmobranchs. Cestodes are transferred according to the host’s feeding ecology throughout all trophic levels. Analyses of stomach contents and parasites in combination can be of further assistance to understand marine food webs and diet availabilities in different habitats, for a variety of host species and their ecology.
The shown dissimilarities in the present study might be either referred to a different degree of pollution or variances in environmental conditions in general. In Penyu Bay these differences might be caused by the nearby city Cilacap and its industries or are resulting from a different feeding ecology, e.g., differences of the examined cartilaginous elasmobranchs, reflecting the different roles of intermediate host for the occurrence of the trypanorhynch cestodes at the sampling sites. Our results demonstrate that the trypanorhynch fauna varies within the studied vulnerable and endangered elasmobranchs, while the feeding ecology is an influencing factor for their composition. Such studies will shed more light onto the scarce knowledge on the elasmobranchs ecology and can be seen as a future baseline for the health status and conservation studies of Indonesian sharks and rays.
Data Availability Statement
Data would be made available on reasonable request.
References
Blaber SJM, Dichmont CM, White W, Buckworth R, Sadiyah L, Iskandar B, Nurhakim S, Pillans R, Andamari R, Dharmadi F (2009) Elasmobranchs in southern Indonesian fisheries: the fisheries, the status of the stocks and management options. Rev Fish Biol Fisheries 19(3):367–391. https://doi.org/10.1007/s11160-009-9110-9
Fahmi D (2015) Pelagic shark fisheries of Indonesia’s Eastern Indian Ocean fisheries management region. Afr J Mar Sci 37(2):259–265. https://doi.org/10.2989/1814232X.2015.1044908
White WT, Last PR, Stevens JD, Yearsley GK, Fahmi, Dharmadi (2006) Economically important sharks & rays of Indonesia. Australian Centre for International Agricultural Research, 330 pp. DOI: https://doi.org/10.22004/ag.econ.114072
Yulianto I, Booth H, Ningtias P, Kartawijaya T, Santos J, Sarmintohadi, Kleinertz S, Campbell S, Palm HW, Hammer C (2018) Practical measures for sustainable shark fisheries: lessons learned from an Indonesian targeted shark fishery. PlosOne, PONE-D-17–42993. https://doi.org/10.1371/journal.pone.0206437
Ferretti F, Myers RA, Serena F, Lotze HK (2008) Loss of large predatory sharks from the Mediterranean Sea. Conserv Biol 22(4):952–964. https://doi.org/10.1111/j.1523-1739.2008.00938.x
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315(5820):1846–1850. https://doi.org/10.1126/science.1138657
Stevens JD, Bonfil R, Dulvy NK, Walker PA (2000) The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci 57(3):476–494. https://doi.org/10.1006/jmsc.2000.0724
Dent F, Clarke S (2015) State of the global market for shark products. FAO Fisheries and Aquaculture Technical paper No. 590: 187 pp.
Hutchinson MR, Itano DG, Muir JA, Holland KN (2015) Post-release survival of juvenile silky sharks captured in a tropical tuna purse seine fishery. Mar Ecol Prog Ser 521:143–154. https://doi.org/10.3354/meps11073
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LNK, Fordham SV, Francis MP, Pollock CM, Simpfendorfer CA, Burgess GH, Carpenter KE, Compagno LJV, Ebert DA, Gibson C, Heupel MR, Livingstone SR, Sanciangco JC, Stevens JD, Valenti S, White WT (2014) Extinction risk and conservation of the world’s sharks and rays. eLife 1–34. https://doi.org/10.7554/eLife.00590.001
Duncan KM, Holland KN (2006) Habitat use, growth rates and dispersal patterns of juvenile scalloped hammerhead sharks Sphyrna lewini in a nursery habitat. Mar Ecol Prog Ser 312:211–221. https://doi.org/10.3354/meps312211
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297. https://doi.org/10.3354/meps337287
Mourier J, Mills SC, Planes S (2013) Population structure, spatial distribution and life-history traits of blacktip reef sharks Carcharhinus melanopterus. J Fish Biol 82(3):979–993. https://doi.org/10.1111/jfb.12039
Sleeman JC, Meekan MG, Wilson SG, Polovina JJ, Stevens JD, Boggs GS, Bradshaw CJA (2010) To go or not to go with the flow: environmental influences on whale shark movement patterns. J Exp Mar Biol Ecol 390(2):84–98. https://doi.org/10.1016/j.jembe.2010.05.009
Blackburn DG (2000) Classification of the reproductive patterns of amniotes. Herpetol Monogr 14:371–377. https://doi.org/10.2307/1467051
Dulvy NK, Reynolds JD (1997) Evolutionary transitions among egg-laying, live-bearing and maternal inputs in sharks and rays. Proc R Soc Lond 264:1309–1315. https://doi.org/10.1098/rspb.1997.0181
Last PR, White WT, Motomura H (2007) Part 6—A description of Squalus chloroculus sp. nov., a new spurdog from southern Australia, and the resurrection of S. montalbani Whitley. CSIRO Marine Atmos Res Paper 14:55–69
Compagno LJV (1984) FAO Species Catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1—Hexanchiformes to Lamniformes. FAO Fisheries Synopsis, Rome, FAO. 125(4/1): 1–249.
Mucientes GR, Queiroz N, Sousa LL, Tarroso P, Sims DW (2009) Sexual segregation of pelagic sharks and the potential threat from fisheries. Biol Let 5(2):156–159. https://doi.org/10.1098/rsbl.2008.0761
Stevens J (2003) The IUCN Red List of Threatened Species. SSG Australia & Oceania Regional Workshop, March 2003. Centroselachus crepidater. e.T46864A11087086. http://dx.doi.org/https://doi.org/10.2305/IUCN.UK.2003.RLTS.T46864A11087086.en
Smith SE, Au DW, Show C (2008) Camhi MD, Pikitch EK, Babcock EA (eds). Pitcher TJ (Series eds). Intrinsic rates of increase in pelagic elasmobranchs. Sharks of the open ocean: biology, fisheries and conservation. Wiley, Blackwell. ISBN: 9780632059959, 9781444302516, 535 pp.
Bornatowski H, Braga RR, Abilhoa V, Corrêa MFM (2014) Feeding ecology and trophic comparisons of six shark species in a coastal ecosystem off southern Brazil. J Fish Biol 85(2):246–263. https://doi.org/10.1111/jfb.12417
Braga RR, Bornatowski H, Vitule JRS (2012) Feeding ecology of fishes: an overview of worldwide publications. Rev Fish Biol Fisheries 22(4):915–929. https://doi.org/10.1007/s11160-012-9273-7
Viana AF, Valentin JL, Vianna M (2017) Feeding ecology of elasmobranch species in southeastern Brazil. Neotropical Ichthyology 15(2):1–13. https://doi.org/10.1590/1982-0224-20160176
IUCN (2017) The IUCN Red List of Threatened Species. Version 2017–3. <http://www.iucnredlist.org>. Downloaded on 05 December 2017
Palm HW, Yulianto I, Piatkowski U (2017) Trypanorhynch assemblages indicate ecological and phylogenetical attributes of their elasmobranch final hosts. Fishes 2:8. https://doi.org/10.3390/fishes2020008
Palm HW (2011) Fish parasites as biological indicators in a changing world: can we monitor environmental impact and climate change? In: Mehlhorn H. (ed) Progress in parasitology, Springer, Berlin/Heidelberg, Germany, pp 223–250. DOI: https://doi.org/10.1007/978-3-642-21396-0_12
Hermosilla C, Prieto R, Silva LMR, Kleinertz S, Taubert A, Silva Monica A (2015) Endo- and ectoparasites of large whales (Cetartiodactyla: Balaenopteridae, Physeteridae): overcoming difficulties in obtaining appropriate samples by non- and minimally-invasive methods. Int J Parasitol: Parasites Wildl 4:414–420. https://doi.org/10.1016/j.ijppaw.2015.11.002
HW Palm 2004 The Trypanorhyncha diesing, 1863 PKSPL-IPB Press Bogor, Indonesia, ISBN: 9799336392, 9789799336392. 710 pp
Palm HW, Rückert S (2009) A new approach to visualize ecosystem health by using parasites. Parasitol Res 105:539–553. https://doi.org/10.1007/s00436-009-1423-z
Sastranegara MH, Fermon H, Mühlenberg M (2003) Technological and institutional innovations for sustainable rural development. Diversity and abundance of intertidal crabs at the east swamp-managed areas in Segara Anakan, Cilacap, Central Java, Indonesia. Deutscher Tropentag, 8–10, October, Göttingen, Germany.
Romimohtarto K, Hutagalung H, Razak H (1991) Water quality of Segara Anakan-Cilacap (Central Java, Indonesia) with a note on lagoon fishery. In: Chou LM, Chua TE, Khoo HW, Lim PE, Paw JN, Silvestre GT, Valencia MJ, White AT, Wong PK (eds) Towards an integrated management of tropical coastal resources. International Center for Living Aquatic Resources Management Conference Proceedings 22: pp. 131–141.
Jennerjahn TC, Yuwono E (2009) Segara Anakan, Java, Indonesia, a mangrove-fringed coastal lagoon affected by human activities. Reg Environ Change 9(4):231–233. https://doi.org/10.1007/s10113-009-0089-5
Palm HW, Rückert S (2009) Metazoan fish parasites of Segara Anakan Lagoon, Indonesia, and their potential use as biological indicators. Reg Environ Change 9:315–328. https://doi.org/10.1007/s10113-008-0076-2
Proctor SP, Merta IGS, Sondita MFA, Wahju RI, Davis TLO, Gunn JS, Andamari R (2003) A review of Indonesia’s Indian Ocean Tuna Fisheries. ACIAR Project FIS/2001/079. 106 pp.
Carpenter KE, Niem VH (1998) The living marine resources of the Western Central Pacific Vol. 2 Cephalopods, crustaceans, holothurians and sharks. Food and Agricultural Organization of the United Nations, Rome; 687–1396.
Last PR, White WT, Carvalho MR, de Séret B, Stehmann MFW, Naylor GJP (eds) (2016) Rays of the World. CSIRO Publishing, Melbourne. 832 pp. DOI: https://doi.org/10.1071/9780643109148
Cribb TH, Bray RA (2010) Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst Parasitol 76:1–7. https://doi.org/10.1007/s11230-010-9229-z
Khalil LF, Jones A, Bray RA (1994) Keys to the cestode parasites of vertebrates. CAB International, Wallingford, U.K. 751 pp. ISBN: 9780851988795
Bush AO, Lafferty KD, Lotz JM (1997) Shostak AWParasitology meets ecology on its own terms: Margolis et al revisisted. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Hyslop EJ (1980) Stomach contents analysis—a review of methods and their application. J Fish Biol 17:411–429. https://doi.org/10.1111/j.1095-8649.1980.tb02775.x
Klimpel S, Seehagen A, Palm HW (2003) Metazoan parasites and feeding behaviour of four small-sized fish species from the central North Sea. Parasitol Res 91:290–297. https://doi.org/10.1007/s00436-003-0957-8
Pinkas L, Oliphant MD, Iverson ILK (1971) Food habits of albacore, bluefin tuna and bonito in Californian waters. Calif Fish Game 152:1–105
Kruskal JB (1964) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:115–129. https://doi.org/10.1007/BF02289694
Kruskal JB, Wish M (1978) Multidimensional scaling. In: Sage University Paper series on Quantitative Applications in the Social Sciences, Sage Publications, Beverly Hills, CA, USA; London, UK, 96 pp. ISBN: 9780585212241
Field JG, Clarke KR, Warwick RM (1982) A practical strategy for analysing multispecies distribution patterns. Mar Ecol Prog Ser 8:37–52. https://doi.org/10.3354/meps008037
Bell JJ, Barnes KA (2003) Effect of disturbance on assemblages: an example using Porifera. Biol Bull 205:144–159. https://doi.org/10.2307/1543235
Mudry DR, Dailey MD (1971) Postembryonic development of certain tetraphyllidean and trypanorhynchan cestodes with a possible alternative life cycle for the order Trypanorhyncha. Can J Zool 49:1249–1253. https://doi.org/10.1139/z71-188
Dollfus RP (1942) Études critiques sur les tétrarhynques du Muséum de Paris. Archives du Muséum National d‘Histoire Naturelle, 6. Série 19:7–466
Mattis TE (1986) Development of two tetrarhynchidean cestodes from the northern Gulf of Mexico. Dissertation, University Southern Mississippi, 171 pp.
Owens L (1981) Relationships between some environmental parameters and trypanorhynch cestode loads in banana prawns (Penaeus merguiensis de Man). Aust J Mar Freshw Res 32:469–474. https://doi.org/10.1071/MF9810469
Kinney MJ, Hussey NE, Fisk AT, Tobin AJ, Simpfendorfer CA (2011) Communal or competitive? Stable isotope analysis provides evidence of resource partitioning within a communal shark nursery. Mar Ecol Prog Ser 439:263–276. https://doi.org/10.3354/meps09327
Vaudo JJ, Heithaus MR (2011) Dietary niche overlap in a nearshore elasmobranch mesopredator community. Mar Ecol Prog Ser 425:247–260. https://doi.org/10.3354/meps08988
Ebert DA, Fowler S, Compagno L (2013) Shark of the World. Illustrated by Marc Dando. Wild Nature Press, 608 pp. ISBN: 9780691205991
Acknowledgements
We are thankful to the Indonesian State Ministry of Research and Technology (RISTEK) for the support and research permit in 2009 (No. Surat Izin to Sonja Kleinertz: 0037/FRP/SM/II/09). We are thankful for institutional support to the Leibniz Center for Tropical Marine Ecology, GmbH, Bremen, Germany, and the Jenderal Soedirman University (UNSOED), Purwokerto, Java. We also acknowledge the SPICE II Project funded by the German Ministry for Education and Research (BMBF), Grant No. 03F0471, for providing study samples and materials. Special thanks to Mr Andih Rinto Suncoko and Mr Edwin Hermawaran, former students from UNSOED University, for their personal initiative and organizational support during fieldwork. We would also like to thank the Department of Zoology at the University of Duesseldorf for the support in electron microscopic studies. This is publication no. 16 under the Memorandum of Understanding between the Faculty of Veterinary Medicine, Udayana University, Bali, and the Faculty of Agricultural and Environmental Sciences, Aquaculture and Sea-Ranching, University of Rostock, Germany, in order to promote fish parasite and biodiversity research in Indonesia.
Funding
Open Access funding enabled and organized by Projekt DEAL. We are thankful to the Indonesian State Ministry of Research and Technology (RISTEK) for the support and research permit in 2009 (No. Surat Izin to Sonja Kleinertz: 0037/FRP/SM/II/09). We are thankful for institutional support to the Leibniz Center for Tropical Marine Ecology, GmbH, Bremen, Germany, and the Jenderal Soedirman University (UNSOED), Purwokerto, Java. We also acknowledge the SPICE II Project funded by the German Ministry for Education and Research (BMBF), Grant No. 03F0471, for providing study samples and materials.
Author information
Authors and Affiliations
Contributions
SoKl: Conception, writing and editing of manuscript, writing of intro and discussion, partly writing of conservation related text, compilation of all data in tables, writing material and method (m & m) parts 2.3-2.4, Supervision of practical part of the thesis in Indonesia 2009 (C. Kurschat); IY: Performance of statistics and analyses, MDS plots, incl. texts for those (mds parasites and stomach contents, 3.2-3.3), partly writing of conservation related text, correction of final draft; CK: Fish parasite isolation, parasite identification and all analyses (lab work), writing of abstract (as part of her master thesis); SvKo: Correction of manuscript, photo plate development; BMS: Writing of ecological role of elasmobranchs, validation and review of identification of all elasmobranch species, partly writing of conservation related text; SvKl: Advisor of master thesis (C. Kurschat), providing of material, correction of final draft; ST: Writing of m & m part: 2.1–2.2, correction of final draft; PU: Writing of result part: 3.1-3.3, correction of final draft, partly writing of discussion; HR: Support of compilation of the tables, writing of MDS result texts; XN-D: Sighting and (re)identification of Trypanorhyncha, correction of manuscript; DB: Editing of manuscript, English correction of the final draft; IMD: Providing lab space for the study at UDAYANA University, Bali; HWP: Advisor and revisor of master thesis (C. Kurschat), providing material, discussion of results, correction of final draft, sighting and (re)identification of Trypanorhyncha, editing of manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Patient Consent Statement
Not applicable.
Permission to Reproduce Material from Other Sources
Not applicable.
Clinical Trial Registration
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kleinertz, S., Yulianto, I., Kurschat, C. et al. Elasmobranchs from Indonesian Waters: Feeding Ecology and Trypanorhynch Cestode Fauna Composition to Support Efforts in Shark and Ray Conservation. Acta Parasit. 67, 1612–1625 (2022). https://doi.org/10.1007/s11686-022-00593-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00593-7