Abstract
An extensive parasitic survey on the Black Pomfret Parastromateus niger (Bloch) (Carangidae) was carried out along the coastal waters of India. A total of 162 host fish (P. niger) were collected, in which 72.2% of the fish were infested with parasitic crustaceans. Six species of parasitic crustaceans were collected, including one species of cymothoid isopod Cymothoa eremita (Brünnich, 1783), and five species of copepods belonging to five different families such as Bomolochus megaceros Heller, 1865 (Bomolochidae), Lernaeenicus stromatei Gnanamuthu, 1953 (Pennellidae), Lernanthropus koenigii Steenstrup and Lütken, 1861 (Lernanthropidae), Synestius caliginus Steenstrup and Lütken, 1861 (Caligidae) and Thysanote appendiculata. (Steenstrup and Lütken, 1861) (Lernaeopodidae). Even though six species of parasitic crustaceans were recovered from the host fish, simultaneous multiple parasitism seldom occurred (only 10%). The prevalence, mean intensity, and mean abundance of each parasite were calculated. The taxonomic account, host, site, and niche-specific parasitization of each species, are discussed. The present study also reviewed and discussed the simultaneous multiple co-occurrence of parasitic crustaceans.
Similar content being viewed by others
Introduction
The fishery sector in India plays a major role in the country's socio-economy. The black pomfret, Parastromateus niger (Bloch), the only known member of the genus Parastromateus Bleeker (Carangidae), is commercially harvested in India. This pelagic fish species, often occurring in large schools in depths of 15–40 m, generally over muddy bottoms (Yadollahvand and Rahnama 2014; Froese and Pauly 2023; Fricke et al. 2023). It is broadly distributed across the Persian Gulf and the Oman Sea, tropical, subtropical, and temperate seas of the world, the Indian and the Pacific Oceans, China and the Malay Archipelago and most abundant on the west coast of India and Indonesia (Froese and Pauly 2023; Fricke et al. 2023). Information regarding the parasitic infestation of swift-swimming fishes in the open water is scanty. Few reports are available about the parasitic crustaceans infesting P. niger, and the buccal cavity, operculum, gill apparatus and body surface of P. niger are common niches for ectoparasites (Pillai 1985).
The great majority of parasitic crustaceans on marine fish belong to groups such as Copepoda and Isopoda (Margolis et al. 1982; Oktener and Sezgin 2000; Maran et al. 2012). Reports showed that marine fishes are highly susceptible to parasitization with copepods and isopods (Pillai 1985; Helna et al. 2016, 2018, 2023; Aneesh et al. 2021a, b, 2022a, b; Nashad et al. 2022). Many marine fishes are known to host more than one species of parasitic crustaceans (Yamaguti and Yamasu 1959; Ohtsuka et al. 2009; Aneesh et al. 2023a), even though a comprehensive study involving the simultaneous infestation, prevalence, site, and niche specificity of each parasitic species is highly necessary. The present study addressed in detail the ectoparasitic crustacean infestation on P. niger from Indian waters with special reference to the host specificity, site specificity and niche separation of each species recovered. The rate of infestation on host fish in terms of prevalence, mean intensity and mean abundance are discussed in detail. The present study also reviewed and discussed the simultaneous multiple co-occurrence of parasitic crustaceans.
Materials and Methods
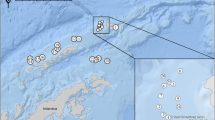
A total of 162 fish, P. niger were collected and examined from February 2016 to January 2021 as part of the routine sampling. Collected specimens of Black pomfret P. niger were collected from various fish landing centers from different localities (Fig. 1) along the Kerala coast of the Arabian Sea (Bekal, 12°24′05''N, 75°00′45''E; Azhikkal, 11°56′36"N, 75°18′36"E; Ayyikkara, 11°51′30"N, 75°22′27"E; Perumatura, 8°37′40''N; 76°47′16''E; Ponnani, 10°46′57.9"N, 75°54′32"E), the southwest coast (Muttom, 8°07′48" N, 77°19′12"E); the Chennai Coast in the Bay of Bengal (Marina Beach, 13°02′57"N, 80°16′58"E) and Coromandel Coast. Soon after collection, the fish were thoroughly examined (general body surface, lateral line region, the base of the pectoral fin, near operculum and posterior-ventral sides, branchial cavity, gill filaments, inner wall of operculum, buccal cavity, etc.) for the presence of parasitic crustaceans. Recovered specimens were preserved in 90% ethanol. Methods for preservation, dissection, and mounting for identification were according to the techniques described in Aneesh et al. (2018, 2023b). The identification was performed using a dissection microscope and microphotographed by using multi focusing stereo microscope Leica-M205A, according to Pillai (1985) and Martin et al. 2016. The prevalence (P), mean intensity (I), and mean abundance were calculated according to Margolis et al. (1982) and Bush et al. (1997). Since we depended on fish markets for the study, the actual parasitism may have been underestimated, since during the time of processing, some pre-adult stages of parasitic copepods may have been lost. Sources for the fish taxonomy and host nomenclature were based on Fish Base (Froese and Pauly 2023) and Catalogue of Fishes (Fricke et al. 2023). The body length of the cymothoid is measured from the cephalon to the distal margin of the pleotelson. The total length of the copepods excludes egg strings. Mean and standard deviation are calculated in Excel, and the total number is given (N).
Map illustrating the location of sampling sites of the Black pomfret Parastromateus niger (Carangidae), along the Kerala coast of the Arabian Sea (Bekal, 12°24′05''N, 75°00′45''E; Azhikkal, 11°56′36"N, 75°18′36"E; Ayyikkara, 11°51′30"N, 75°22′27"E; Perumatura, 8°37′40''N; 76°47′16''E; Ponnani, 10°46′57.9"N, 75°54′32"E), the southwest coast (Muttom, 8°07′48" N, 77°19′12"E), and the Chennai Coast in the Bay of Bengal (Marina Beach, 13°02′57"N, 80°16′58"E) (source: https://www.google.co.in/maps)
Results
A total of 162 P. niger were collected and examined. More than 70% (117 out of 162) of the examined fishes were infested with parasitic crustaceans. Six species of parasitic crustaceans, including one cymothoid isopod, Cymothoa eremita (Brünnich, 1783), and five species of copepods from five different families, i.e. Lernaeenicus stromatei Gnanamuthu 1953 (Pennellidae), Bomolochus megaceros Heller, 1865 (Bomolochidae), Lernanthropus koenigii Steenstrup and Lütken, 1861 (Lernanthropidae), Synestius caliginus Steenstrup and Lütken, 1861. (Caligidae) and Thysanote appendiculata (Steenstrup and Lütken, 1861) (Lernaeopodidae) were found to be using P. niger as a host (Figs. 2, 3, 4 and 5). A total of 357 parasitic copepods, including 148 B. megaceros, 31 S. caliginus, 8 (+ 2 males attached with females) L. koenigii, 41 T. appendiculata, 134 L. stromatei, and 30 individuals of cymothoid C. eremita, were recovered from the infested host fishes (Table 1).
Parasitic crustacean infesting the Black Pomfret, Parastromateus niger (Bloch, 1795). a Parastromateus niger; b Cymothoa eremita (Brünnich, 1783) - female; c Synestius caliginus Steenstrup & Lütken, 1861 - female; d Bomolochus megaceros Heller, 1865 - female; e Thysanote appendiculata (Steenstrup & Lütken, 1861)- female; f Lernanthropus koenigii Steenstrup & Lütken, 1861 - female; g Lernaeenicus stromatei Gnanamuthu, 1953 - female
Systematic account
Order Isopoda Latreille, 1817
Suborder Cymothoida Wägele, 1989
Superfamily Cymothooidea Leach, 1814
Family Cymothoidae Leach, 1814
Genus Cymothoa Fabricius, 1793
Syn. (See Aneesh et al. 2022b).
Cymothoa eremita (Brünnich, 1783)
Syn. (See Martin et al. 2016).
The buccal attaching C. eremita, is a widely known species and it can be identified by the following combinations of characters: 1) anterolateral margins of pereonite 1 extending nearly half the length of cephalon; 2) subtruncate cephalon; 3) small horn-like structures on the posterolateral margins of pereonite 1; 4) pleon as wide as pereon; 5) uropods not extending to pleotelson posterior margin; 6) pereopod 7 with a bulbous protrusion on ischium. It was recovered from the buccal cavity of the black pomfret, Parastromateus niger, over the tongue. Among 162 fish (P. niger) examined, 29 were found to be parasitized with Cymothoa eremita; the prevalence and mean intensity being 17.9% and 1.03, respectively (Table 1). Except for one male, all other recovered parasites (C. eremita) were ovigerous females with or without brood. Females were attached to the floor of the buccal cavity, their cephalon facing towards the opening of the host mouth; the male settled just behind the female on the gill arch.
Size: ovigerous females, 27.87 ± 2.92 mm (N = 29), male 8.00 mm (N = 1).
Colour: ovigerous female and male-tan colour.
Other host records: recorded from P. niger (as Stromateus niger); Psettodes erumei (Bloch and Schneider); Liza vaigiensis (Quoy and Gaimard) (as Mugil waigiensis) and Peprilus paru (Linnaeus) (as Stomateus paru); Psuedanthias evansi (Smith); Arothron leopardus (Day) (as Tetrodon leopardus); Tetrodon sp.; Hime formosana (Lee and Chao) (as Aulopus japonicus); Parastromateus sp. and Psettodes sp.; Pampus argenteus (Euphrasen) (as Stromateus cinereus); Siganus canaliculatus (Park) (as S. oramin); Plectorhinchus nigrus (Cuvier) (as Pseudopristipoma nigrum); Sphyraena obtusata Cuvier (see Martin et al. 2016 for detailed references).
Distribution: Cymothoa eremita is widely distributed in the Indian Ocean and Indo-Pacific, Japan –Pescsdores, the Philippines and Indonesia – the Cape York Peninsula, Australia, and East to the Society island, Singapore, Malaysia and Bangkok, Ceylon, Arabian Gulf, Indian Peninsula from Madras to Bombay, Mauritius, Seychelles and the Red sea; Japan; and Arabian Gulf; South China Sea; and the Philippines, Hawaii; from southwest coast, Kerala coast, Bay of Bengal and Coromandel Coast of West Bengal, India (see Martin et al. 2016).
Subclass Copepoda Milne Edwards, 1840
Order Cyclopoida Burmeister, 1834
Family Bomolochidae Claus, 1875
Genus Bomolochus von Nordmann, 1832
Bomolochus megaceros Heller, 1865
a–b Bomolochus megaceros Heller, 1865 ex Parastromateus niger (Bloch), a site of attachment; b ovigerous female; c–d Synestius caliginus Steenstrup & Lütken, 1861 ex Parastromateus niger (Bloch), c ovigerous female; d male; e–f Lernanthropus koenigii Steenstrup & Lütken, 1861 ex Parastromateus niger (Bloch), e. ovigerous female; f male; g–h Thysanote appendiculata (Steenstrup & Lütken, 1861) ex Parastromateus niger (Bloch), g non-ovigerous female; h ovigerous female
Syn. (See Pillai 1985).
The genus Bomolochus consists of 21 valid species, among them five species have been previously reported from India, including B. megaceros (Pillai 1985; Izawa 2020). This species can be recognized by its comparatively large body and by the long slender, nearly straight processes on the antennule. Bomolochus megaceros (Fig. 4b) was recovered from the inner opercular fold of the host fish. Fifty nine host fish out of 162 examined were infested with B. megaceros and a total of 148 females were recovered; the prevalence and intensity being 36.4% and 2.5 respectively (Table 1). Interestingly all the recovered members were found to be reproductively active females possessing egg sacs and/or maturing ovaries. It also exhibited site and niche-specific parasitization infesting the inner side of the operculum (Fig. 4a), as do several other parasitic crustaceans.
Size: female, 2.86 ± 0.12 mm (N = 148).
Colour: white.
Other host records: P. niger, Caranx djedaba (= Alepes djedaba (Forsskål)), Stromatoides sinensis Forster, Caranx sp., Mugil sp., Therapon theraps Cuvier, Megalaspis cordyla (Linnaeus) (see Pillai 1985).
Distribution: from southwest coast, Kerala coast, Bay of Bengal and Coromandel Coast of West Bengal, Bombay Coast, India, Sri Lanka, South China Sea, China, Baluchistan Taiwan (Pillai 1985).
Order Siphonostomatida Burmeister, 1835
Family Caligidae Burmeister, 1835
Genus Synestius Steenstrup and Lütken, 1861
Synestius caliginus Steenstrup and Lütken, 1861
Syn. (See Pillai 1985).
The genus Synestius is a monotypic genus with S. caliginus Steenstrup and Lütken, 1861 and have previously been reported from India (Pillai 1985). The female S. caliginus can be identified by the following combinations of features: the presence of two pairs of long club-shaped processes on the genital complex; genital segment enlarged and subequal to cephalothorax in size; abdomen long, club-shaped and two-segmented, longer than processes of genital segment. Seventeen out of 162 observed host fish were infested by Synestius caliginus with a total of 30 females and 1 male; the prevalence being 10.5. Synestius caliginus also exhibits the site specific parasitization, collected from the gill mucous and opercular region of the host.
Size: ovigerous female, 4.61 ± 0.08 mm (N = 30); male, 3.60 mm (N = 1).
Colour: female and male are colorless.
Other host records: P. niger, Pampus argenteus (Euphrasen), Stromatoides sinensis (Pillai 1985).
Distribution: Batavia, Java, Malaysia, Sri Lanka, Persian Gulf, China, Taiwan, from southwest coast, Kerala coast, Bay of Bengal and Coromandel coast of West Bengal, India (Steenstrup and Lütken 1861; Pillai 1985; Present study).
Family Lernanthropidae Kabata, 1979
Genus Lernanthropus de Blainville, 1822
Lernanthropus koenigii Steenstrup and Lütken 1861
Syn. (See Pillai 1985).
To date the genus Lernanthropus de Blainville, 1822 consists of 103 valid species, among them about 26 species have previously been reported from India including Lernanthropus koenigii Steenstrup and Lütken 1861 (Pillai 1985; Boxshall et al. 2020). Lernanthropus koenigii collected from the gill filaments. This species can be identified by the following combinations of characters: cephalothorax nearly as long as wide; antennular lobe half the width of frontal margin; dorsal plate longer but narrower than anterior division of trunk, nearly equal in length and width, postero-medially concave; genital segment large, longer than broad; abdomen partially fused with genital segment; caudal rami only slightly shorter than abdomen. The lowest prevalence (P = 3.7%) among the recovered parasitic crustaceans is shown by L. koenigii; recovered from only six hosts out of 162 fish and 8 females and 2 males were recovered with the intensity being 1.3. All the eight females were reproductively active, evidenced by the presence of growing ovaries and/or egg sacs. The recovered males were found clinging on the posterior part of the female body in a copulatory position, especially attaching to the genital complex of the females using its maxilliped (these males were not included for calculation of prevalence and mean intensity).
Size: female 4.43 ± 0.07 mm (N = 8), male 2.20, 2.30 mm (N = 2).
Colour: in live condition red in colour.
Other host records: Stromateus paru (= Peprilus paru (Linnaeus)) (Pillai 1985).
Distribution: According to Pillai (1985), Tranquebar, India (Madras, Kerala), southwest coast, Kerala coast, Bay of Bengal and Coromandel coast of West Bengal, India (present study).
Family Lernaeopodidae Milne Edwards, 1840
Genus Thysanote Krøyer, 1863
Thysanote appendicSmyulata (Steenstrup and Lütken, 1861)
Syn. (See Pillai 1985).
According to Walter and Boxshall (2022) 20 valid species of Thysanote have been reported worldwide among them four species have previously been reported from India (Pillai 1985; Piasecki et al. 2008). Thysanote appendiculata can be identified by the presence of two dichotomously branched processes on each maxilla and each hind corner of trunk with two processes. Out of 162 fish, 32 were infested by T. appendiculata. A total of 41 mature/immature females were recovered from 32 infested hosts; the prevalence and intensity being 19.8 and 1.3, respectively (Table 1). It was recovered from the proximal region of the gill rack.
Size: ovigerous female, 5.99 ± 0.52 mm (N = 41).
Colour: colourless/translucent.
Other host records: P. niger is the only reported host.
Distribution: From southwest coast, Kerala coast, Bay of Bengal and Coromandel coast of West Bengal, India (Pillai 1985; Present study).
Family Pennellidae Burmeister, 1835
Genus Lernaeenicus Le Sueur, 1924
Syn. (See Aneesh et al. 2021a).
Lernaeenicus stromatei Gnanamuthu, 1953
Syn. (See Aneesh et al. 2018).
So far 32 valid species of Lernaeenicus have been described, worldwide (Walter and Boxshall 2022) among them ten species have been reported from India (Pillai 1985; Raja et al 2016; Aneesh et al. 2018, 2021a). Lernaeenicus stromatei is identified by the following characters such as, 1) body very long and slender, head, anteriorly rounded and fairly dorso-ventrally flattened and slightly longer than broad (Fig. 5b, c), 2) Three posterior horns, one median and two lateral, all sub-similar and apically rounded, 3) Anterior part of neck with indistinct partition on dorsal side, indicating thoracic segments and a three-segmented antennule. As do other pennellids in the present study L. stromatei also penetrate the flesh of the host fish. Among the parasitic crustaceans recovered in the present study, the second highest prevalence is exhibited by Lernaeenicus stromatei (P = 28.4%). A total of 134 copepods were recovered, the intensity being 2. 9 (Table 1) (Fig. 3). L. stromatei also exhibits a niche specific parasitization, occurring near to opercular bone and gill, burrowed into the body, extending outward through gonopore of the host fish.
Size: ovigerous female, 58.64 ± 10.89 mm (N = 134).
Colour: live specimens are translucent with light red in colour.
Other host records: reported only from P. niger (Gnanamuthu 1953; Pillai 1985; Aneesh 2014; Raja, et al. 2016; Aneesh et al. 2018).
Distribution: Chennai (type locality) (Gnanamuthu 1953; Aneesh et al. 2018, present study), Malabar Coast (Aneesh 2014; present study), West Bengal (present study), South east coast (Raja et al. 2016; Aneesh et al. 2018).
Discussion
Parastromateus niger hosts many parasitic copepod species, listed and described by Pillai (1985). In the present study more than 70% (117 out of 162) of P. niger were infested by parasitic crustaceans. Six species of parasitic crustaceans, including one cymothoid C. eremita and five species of copepods from five different families i.e. B. megaceros, S. caliginus, L. koenigii, T. appendiculata and L. stromatei, were found to be parasitizing P. niger. A total 362 parasitic copepods including 148 B. megaceros, 31 S. caliginus, 8 females (+ 2 males attached with females) L. koenigii, 41 T. appendiculata and 134 L. stromatei and 30 individuals of cymothoid isopod, C. eremita, were collected from the infested host fishes (Table 1). Each parasitic crustacean recovered from P. niger exhibit a strict site and niche specificity. Bomolochus megaceros and L. stromatei were found to be the two major copepod parasites of P. niger with prevalences of 36.4% and 28.4% respectively.
Few reports are available on the simultaneous occurrence of multiple parasitism involving exclusively parasitic crustaceans. Daniel and Rama Rao (1967) reported the simultaneous infestation of another flying fish Parexocoetus mento (Valenciennes) by an external attaching cymothoid isopod, Nerocila sp. and a pennellid copepod, Pennella sp. which was embedded into the body of the host fish. Incidence of double parasitism involving the isopod Nerocila phaiopleura Bleeker, 1857 and the copepod Lernaeenicus sprattae (Sowerby, 1806) was reported in anchovy fish Stolephorus commersonii Lacepède, from Indian waters (Rajkumar et al. 2006). Another marine fish (Hemiramphus far (Forsskål)) also showed simultaneously infestation by the isopod Mothocya plagulophora (Haller, 1880) and the copepod Lernaeenicus hemirhamphi Kirtisinghe, 1932 (Gopalakrishnan et al. 2010).
Heavy simultaneous multiple infestation of parasitic crustaceans was also reported in cartilaginous fishes. Hewitt (1979) reported eight species of parasitic copepods infesting an immature female of white shark, Carcharodon carcharias from Pukerua Bay, near Wellington; including seven species of pandarid copepods such as Pandarus satyrus Dana, 1849 (3 females), Nesippus orientalis Heller, 1865 (15 females), Pandarus bicolor Leach, 1816 (5 females), Echthrogaleus coleoptratus (Guérin-Méneville, 1837) (14 females), E. denticulatus Smith S.I., 1873 (3 females), Dinemoura latifolia (Steenstrup and Lütken, 1861) (9 females, and 1 male), and D. producta (Müller O.F., 1785) (17 females, 3 males) and one species from the family Eudactylinidae, Nemesis lamna lamna Risso, 1826 (61 females and 28 males). Similarly, the multiple infestation of a white shark (C. carcharias) by five different species of pandarid copepods, Dinemoura producta, D. latifolia, Eehthrogaleus coleoptratus, Pandarus bicolor, and Achtheinus oblongus Wilson C.B., 1908 was also observed from Morro Bay, California by Benz et al. (2003).
Even though the cymothoids are usually parasitizing a host fish by a single species, the simultaneous infestation of two isopods was also rarely reported. Williams and Bunkley-Williams (1985) shared the incidence of two different co-occurrences from the Caribbean; 1) co-occurrences of the body surface infesting Anilocra abudefdufi Williams and Williams, 1981 and the branchial infesting Kuna insularis (Williams and Williams 1985) parasitising a single individual host of Abudefduf saxatilis (Linnaeus, 1758), and 2) the external attaching Anilocra acanthuri Williams and Williams, 1981 and the gill infesting Agarna cumulus (Haller, 1880) parasitising Acanthurus chirurgus (Bloch, 1787). Two recent reports on co-occurrence of two cymothoid species includes; co-occurrence of the external attaching Anilocra capensis Leech, 1818 and the buccal-infesting Ceratothoa africanae Hadfield, Bruce and Smit, 2014 on the same individual Pachymetopon blochii (Valenciennes, 1830) from South Africa by Welicky and Smit (2018) and the body surface infesting Anilocra grandmaae Aneesh, Hadfield, Smit and Kumar, 2021 and the branchial infesting Agarna malayi Tiwari, 1952, simultaneously parasitizing the same individual of Tenualosa toli (Valenciennes, 1847) from India (see Aneesh et al. 2022a, b).
Different combinations of species involving the simultaneous multiple infestations were also studied in two belonid fishes from the Malabar coast of India: (1) the banded needle fish, Strongylura leiura (Bleeker, 1850) by one cymothoid isopod, Mothocya renardi (Bleeker, 1857) and three copepods such as Lernanthropus tylosuri Richiardi,1880, Caligodes lacinatus Heller, 1868 and Bomolochus bellones Burmeister, 1833 (see Aneesh et al. 2013), and (2) the spot-tail needlefish, Strongylura strongylura (van Hasselt, 1823) by a cymothoid isopod, Cymothoa frontalis Milne Edward, 1840 and four species of copepods such as L. tylosuri C. lacinatus, B. bellones and Dermoergasilus coleus Cressey and Collette, 1970 (Aneesh et al. 2014).
In the present study the majority of infested P. niger (49%; 57 out of 117) harbors only one species of parasitic crustacean, mostly B. megaceros (44 fishes), rarely C. eremita (8 fish) or L. stromatei (8 fish). Remaining 44% of the host fish (P. niger) (51 out of 117) were also found to be parasitized with any of the two parasitic crustacean species at a time in eight different combinations (see Tables 2 and 3). Among them 41% (21 instances out of 51) were in L. stromatei and T. appendiculata combination, another 10 instances L. stromatei and S. caliginus (see Tables 2 and 3). Simultaneous multiple parasitism (more than two species of parasitic crustaceans in a single fish) was seldom in P. niger. Only 5% (6 out of 117) of infested fishes were simultaneously infested by any of the three parasitic copepods (triple parasitism) in 4 different combinations (see Tables 2 and 3). Quadruple parasitism is very rare in P. niger, only 2.5% (3 out of 117) in three different combinations (see Tables 2 and 3). Multiple parasitism may have been cause by the following factors: migratory routes of the host school, breeding, and distribution of infective stages of parasites etc., even though the occurrence of such phenomena seems to be very rare in the present study and it may be due to the site and niche overlap and reducing inter specific competition.
In conclusion each species recovered exhibited very strict site specific parasitization. Even though six different species of parasitic crustaceans attached to different sites in the host fish (P. niger), the simultaneous multiple parasitization was very rare.
Availability of Data and Materials
Not applicable.
References
Aneesh PT (2014) Studies on parasitic crustaceans infesting the fishes of Malabar Coast. Kannur, Kerala, Kannur University, PhD Thesis, 147
Aneesh PT, Sudha K, Helna AK, Arshad K, Anilkumar G, Trilles JP (2013) Simultaneous multiple parasitic crustacean infestation on banded needlefish, Strongylura leiura (Belonidae) from the Malabar Coast, India. Int J Sci Res Publ 3:367–375
Aneesh PT, Sudha K, Helna AK, Anilkumar G, Trilles JP (2014) Multiple parasitic crustacean infestation on belonid fish Strongylura strongylura. In: I.S. Wehrtmann, R.T. Bauer (eds), Proceedings of the Summer Meeting of the Crustacean Society and the Latin American Association of Carcinology, Costa Rica, July 2013. ZooKeys 457:339–353. https://doi.org/10.3897/zookeys.457.6817
Aneesh PT, Helna AK, Bijukumar A, Maran BAV (2018) Redescription of Lernaeenicus stromatei Gnanamuthu, 1953 (Copepoda: Siphonostomatida: Pennellidae) infesting the Black Pomfret Parastromateus niger (Bloch) from Indian waters. Zootaxa 4482(2):375–382. https://doi.org/10.11646/zootaxa.4482.2.9
Aneesh PT, Bruce NL, Kumar AB, Bincy MR, Sreenath TM (2021a) A taxonomic review of the buccal-attaching fish parasite genus Lobothorax Bleeker, 1857 (Crustacea: Isopoda: Cymothoidae) with description of a new species from southwestern India. Zool Stud 60:(13)1‒13. https://doi.org/10.6620/ZS.2021.60-13
Aneesh PT, Hadfield KA, Smith NJ, Biju Kumar A (2021b) Morphological description and molecular characterisation of a new species of Anilocra Leach, 1818 (Crustacea: Isopoda: Cymothoidae) from India. Int J Parasitol Parasites Wildl 14:321–328. https://doi.org/10.1016/j.ijppaw.2021.03.007
Aneesh PT, Helna AK, Bijukumar A (2022a) Simultaneous co-occurrence of two species of cymothoids from two different genera on a single host fish. Nauplius 30(e2022013):1–10. https://doi.org/10.1590/2358-2936e2022013
Aneesh PT, Helna AK, Bijukumar A (2022b) Redescription and further report of two buccal attaching fish parasitic cymothoids, Ceratothoa carinata (Bianconi, 1869) and Cymothoa bychowskyi Avdeev, 1979 (Crustacea: Isopoda) with a new record from the southern India Ocean. J Nat Hist 56(16–17):1063–1089. https://doi.org/10.1080/00222933.2022.2099318
Aneesh PT, Helna AK, Kumar BA, Venmathi Maran BA (2023a) Proposal of a new family for Hirodai ohtsukai gen. et sp. nov. (Crustacea: Copepoda) infesting Uranoscopus guttatus Cuvier, 1829 (Perciformes: Uranoscopidae) from the southwest coast of India. J Nat Hist 57(33–36):1495–1515. https://doi.org/10.1080/00222933.2023.2259556
Aneesh PT, Helna AK, Kumar AB, Maran BAV (2023b) A new species of Cardiodectes Wilson C.B., 1917 (Copepoda: Siphonostomatoida: Pennellidae) from Spinyjaw greeneye, Chlorophthalmus corniger Alcock, 1894 off the Indian Ocean. Zootaxa 5369(2):277–291. https://doi.org/10.11646/zootaxa.5369.2.7
Benz GW, Mollet HF, Ebert DA, Davis CR,Van Sommeran SR (2003) Five Species of Parasitic Copepods (Siphonostomatoida: Pandaridae) from the Body Surface of a White Shark Captured in Morro Bay, California. Pac Sci 57(1):39–43. https://doi.org/10.1353/psc.2003.0002
Boxshall GA, Bernot JP, Barton DP, Diggles BK, Yong RQ, Atkinson-Coyle T, Hutson KS (2020) Parasitic copepods of the family Lernanthropidae Kabata, 1979 (Copepoda: Siphonostomatoida) from Australian fishes, with descriptions of seven new species. Zootaxa 4736(1):1–103. https://doi.org/10.11646/zootaxa.4736.1.1
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms. Margolis et al. revised. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Daniel A, Rama Rao KV (1967) Notes on Animal relationships: A flying fish parasitised by an isopod, and a copepod associated with an inquiline cirripede. Curr Sci 36:641
Fricke R, Eschmeyer WN, van der Laan R editor (2023) Catalog of fishes: genera, species, references. [Electronic version accessed 2023 Jan 22] http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Froese R, Pauly D editor (2023) FishBase. World Wide Web electronic publication, version (09/2009). [Accessed 2023 Jan]. www.fishbase.org
Gnanamuthu CP (1953) Three lernaeid copepods parasitic on South Indian fishes. J Parasitol 39:14–21. https://doi.org/10.2307/3274053
Gopalakrishnan A, Rajkumar M, Sun J, Trilles JP (2010) Occurrence of double parasitism on black-barred halfbeak fish from the southeast coast of India. Chin J Oceanol Limnol 28:832–835
Helna AK, Sudha K, Aneesh PT, Piasecki W, Anilkumar G (2016) A case of persisting massive infection of Scomberomorus commerson, a commercially exploited scombrid fish, with Cybicola armatus (Copepoda: Siphonostomatoida: Pseudocycnidae). Acta Parasitol 61(4):836–848. https://doi.org/10.1515/ap-2016-0116
Helna AK, Sudha K, Aneesh PT, Anilkumar G (2018) Caligus cybii (Caligidae, Copepoda) Parasitising the Commercially Exploited Seer Fish, Scomberomorus commerson, from the Malabar Coast (India)- Occurrence and Adaptations. Turkish J Fish Aquat Sci 18:445–455. https://doi.org/10.4194/1303-2712-v18_3_10
Helna AK, Aneesh PT, Kumar AB, Ohtsuka S (2023) Morphological Description and Molecular Characterisation of Glyptothoa gen. nov., a Fish Parasitic Deep-sea Cymothoid (Crustacea: Isopoda) from the Indian Ocean, with four species, including one new species. Zool Stud 62:(51)1‒31. https://doi.org/10.6620/ZS.2023.62-51
Hewitt, (1979) Eight species of parasitic Copepoda on a white shark. N Z J Mar Freshwater Res 13(1):171–171. https://doi.org/10.1080/00288330.1979.9515790
Izawa K (2020) Some new and known species of Bomolochidae (Copepoda, Cyclopoida) parasitic on Japanese actinopterygian fishes, 1–with discussion on sexually dimorphic features in the family. Crustaceana 93(8):891–929. https://doi.org/10.1163/15685403-bja10048
Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA (1982) The use of ecological terms in parasitology (Report of an ad hoc Committee of the American Society of Parasitologists). J Parasitol 68:131–133. https://doi.org/10.2307/3281335
Maran BAV, Moon SY, Oh S-Y, Soh HY, Myoung J-G (2012) Redescription of two Pennellids (Copepoda, Siphonostomatoida) from Korea with a key to species of Peniculus von Nordmann, 1832. Zookeys 243:1–14. https://doi.org/10.3897/zookeys.243.3668
Martin MB, Bruce N, Nowak B (2016) Monograph: Review of the fish-parasitic genus Cymothoa Fabricius, 1793 (Crustacea: Isopoda: Cymothoidae) from Australia. Zootaxa 4119(1):1–72
Nashad M, Aneesh PT, Kumar AB, Bineesh KK (2022) A new species of branchial fish parasitic isopod, Norileca Bruce, 1990 (Crustacea: Isopoda: Cymothoidae), from the Andaman Islands. India Mar Biol Res 17(7–8):669–679. https://doi.org/10.1080/17451000.2021.2011321
Oktener A, Sezgin MB (2000) Mothocya epimerica Costa (1851) (Flabellifera: Cymothoidae), an isopod parasie in the branchial cavities of the Black Sea Silverfish Atherina boyeri Risso, 1810. J Turk Mar Sci 6:23–29
Ohtsuka S, Takami I, Maran BAV, Ogawa K, Shimono T, Fujita Y, Asakawa M, Boxshall GA (2009) Developmental stages and growth of Pseudocaligus fugu Yamaguti, (Copepoda: Siphonostomatoida: Caligidae) host-specific to puffer. J Nat Hist 43:1779–1804
Piasecki W, Ohtsuka S, Yoshizaki R (2008) A new species of Thysanote Kroyer, (Copepoda: Siphonostomatoida: Lernaeopodidae), a fish parasite from Thailand. Acta Ichthyol Piscat 38(1):29–35
Pillai NK (1985) The Fauna of India: Copepod Parasites of Marine Fishes. Zoological Survey of India, Calcutta
Raja K, Saravanakumar A, Gopalakrishnan A, Vijayakumar R, Hwang UW, Venmathi Maran BA (2016) The genus Lernaeenicus Lesueur (Copepoda, Siphonostomatoida, Pennellidae) in India: a checklist with notes on its taxonomy and ecology. Zootaxa 4174(1):192–211
Rajkumar M, Perumal P, Trilles JP (2006) A note on the double parasitism (Copepod and Isopod) in Commerson’s Anchovy fish (India). J Environ Biol 27:613–614
Steenstrup JJS, CF Lütken, CF (1861) Bidrag til kundskab om det aabne havs snyltekrebs og lernæer samt om nogle andre nye eller hidtil kun ufuldstændigt kjendte parasitiske Copepoder. Kongelige Danske Videnskabernes Selskabs Skrifter, Naturhistorisk og Mathematisk Afdeling, Kjöbenhavn (5)5:343–432. pls. 1–15
Walter TC, Boxshall G (2022) World of copepods database. https://www.marinespecies.org/copepoda; https://doi.org/10.14284/356. Accessed 19 Dec 2022
Welicky RL, Smit NJ (2018) Unique co-occurrence of two genera of cymothoid ectoparasitic isopods on the same individual fish host. Afr J Mar Sci 40:467–469
Williams EH, Bunkley-Williams LB (1985) Cuna insularis n. gen. and n. sp. (Isopoda: Cymothoidae) from the gill chamber of the sergeant major, Abudefduf saxatilis (Linnaeus) (Osteichthyes) in the West Indies. J Parasitol 71:209–214
Yadollahvand R, Rahnama B (2014) The age determination of Black Pomfret (Parastromateus niger), based on otolith cross sections in Iranian coast of Oman Sea. J Aquac Res Dev 5:261. https://doi.org/10.4172/2155-9546.1000261
Yamaguti S, Yamasu T (1959) Parasitic copepods from fishes of Japan with description of 26 new species and remarks on two known species. Biol J Okayama Univ 5(3–4):89–165. pls. 1–14. (ix-1959)
Funding
Open Access funding provided by Hiroshima University. This study was partially supported by grants-in-aid from the Japan Society of Promotion of Science (KAKENHI No. 19H03032, awarded to SO; JSPS Bilateral Partnership Program, No. JPJSBP120209924, awarded to SO). The authors thank the partial funding support of Lee Kong Chan Museum of Natural History, National University of Singapore, for the funding support to University of Kerala on deep-sea crustaceans of India.
Author information
Authors and Affiliations
Contributions
AKH and APT conceived and designed the research, collection, identification, photography, writing the original draft. APT, AKH, AB, SO and BAVM, critically reviewed to improve the quality of the manuscript. All authors have proofread and approved the final submission.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Helna, A.K., Aneesh, P.T., Kumar, A.B. et al. Site-specific Parasitism of Crustaceans on the Black Pomfret, Parastromateus niger (Carangidae) from Indian Waters. Thalassas (2024). https://doi.org/10.1007/s41208-023-00655-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41208-023-00655-1