Laws of Thermodynamics

The laws of thermodynamics are a set of physical laws that define a group of physical quantities, such as temperature, energy, and entropy, that characterize thermodynamic systems in thermodynamic equilibrium. The laws also use various parameters for thermodynamic processes, such as thermodynamic work and heat, and establish relationships between them. They state empirical facts that form a basis of precluding the possibility of certain phenomena, such as perpetual motion.
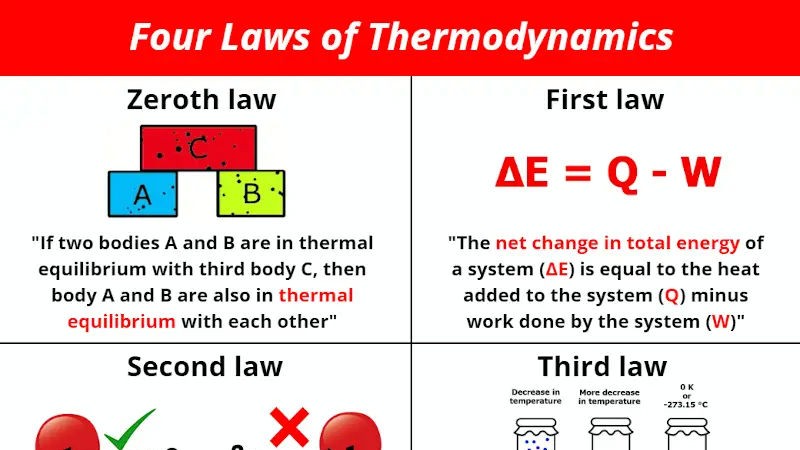
There are four laws of thermodynamics:
Zeroth law of thermodynamics: If two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law defines the concept of thermal equilibrium and allows us to compare the temperatures of different systems.
First law of thermodynamics: The total energy of an isolated system is constant. Energy can be transformed from one form to another, but it cannot be created nor destroyed. This law is a statement of the conservation of energy.
Second law of thermodynamics: The entropy of an isolated system always increases over time. Entropy is a measure of disorder or randomness. This law means that the universe is becoming increasingly disordered over time.
Third law of thermodynamics: The entropy of a perfect crystal at absolute zero (0 K) is zero. Absolute zero is the lowest possible temperature. This law means that it is impossible to reach absolute zero.