Laws of thermodynamics:| Zeroth Law | First Law | Second Law | Third law
The law of thermodynamics are fundamental principles in physics that govern the behavior of energy and matter in the universe. There are four laws of thermodynamics, but the first three are the most well-known and widely applicable. Here are the first four laws of thermodynamics:
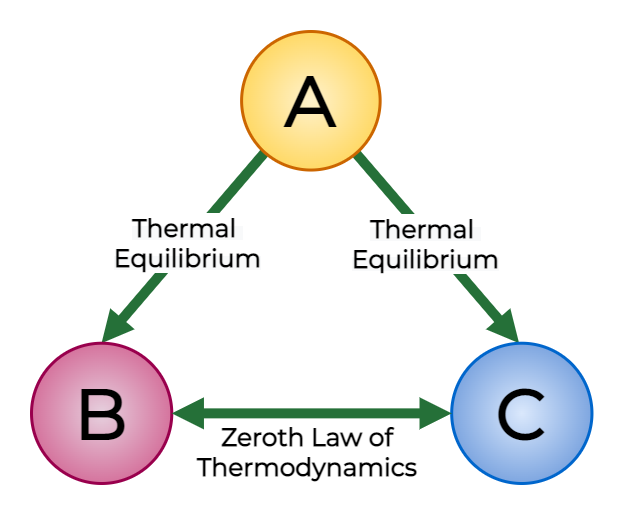
Zeroth Law of Thermodynamics:
If two systems are each in thermal equilibrium with a third system, they are in thermal equilibrium with each other. This law establishes the concept of temperature and allows for the comparison of temperatures between different systems.
The Zeroth Law serves as the basis for temperature measurement and the establishment of thermal equilibrium, which is essential for understanding and applying the other laws of thermodynamics.
First Law of Thermodynamics (Conservation of energy):
Conservation of energy principle says that energy can neither be created nor destroyed, it can only be transferred from one form to another. The sum of all the energies remains constant.
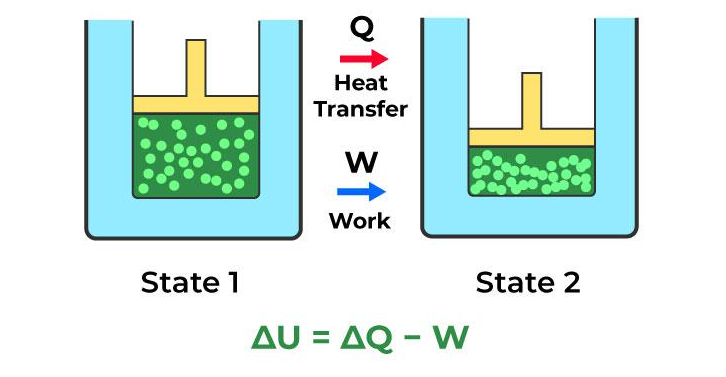
ΔQ = ΔU + W
ΔU =ΔQ – W
ΔQ =Amount of heat energy given.
ΔU =changes in internal energy.
W = work done
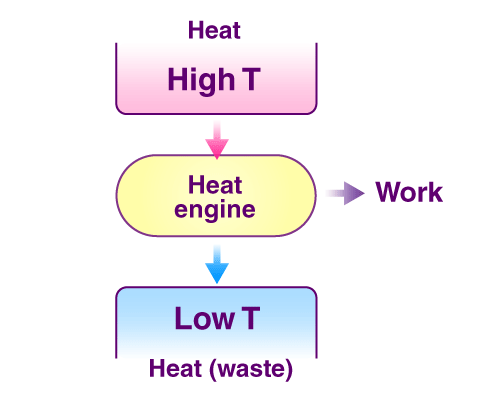
Second law of thermodynamics:
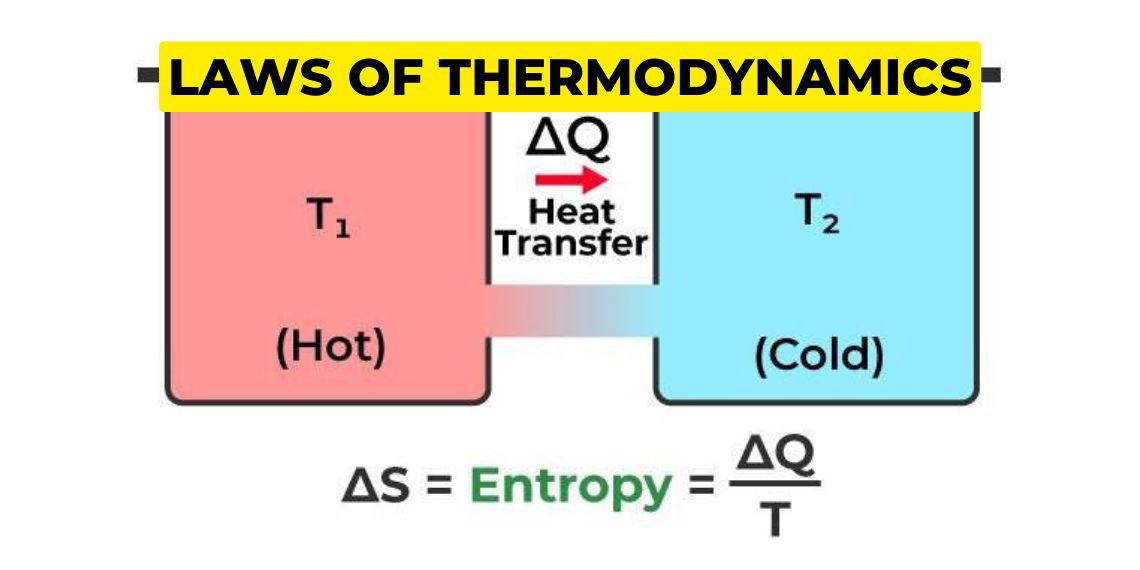
The Second Law of Thermodynamics states that the total entropy of an isolated system can never decrease over time. In simpler terms, it means that any process that occurs in a closed system will always result in an increase in the overall disorder or randomness of the system due to which the usable energy is reduced to do work.
Entropy is a measure of the disorder or randomness of a system. For example, a highly ordered system, such as a crystal, has low entropy, while a disordered system, such as a gas, has high entropy. The Second Law of Thermodynamics states that the natural tendency of any system is to move towards a state of maximum entropy or maximum disorder.
Second law has two statements:
Kelvin Plank statement: It is impossible to construct an engine working on a cyclic process, whose only purpose is to convert heat energy to work without any loss.
Mathematically, the second law of thermodynamics is represented as;
ΔS univ > 0
where ΔS univ is the change in the entropy of the universe.
Third Law of Thermodynamics
As the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius), the entropy of a pure crystalline substance becomes zero. This law sets a fundamental limit on how low temperatures can be achieved.
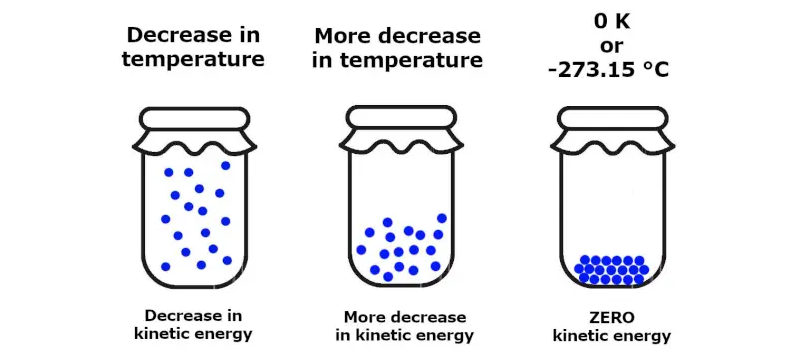
The Third Law implies that it is impossible to reach absolute zero in a finite number of steps. However, it serves as a fundamental reference point for temperature scales. For instance, the Kelvin scale sets absolute zero as the point at which no more heat energy can be extracted from a system, making it the lowest possible temperature.
This law provides a framework for understanding the behaviour of materials at extremely low temperatures and has important implications in fields such as quantum mechanics and the study of phase transitions.
Note:
If you want to learn more about this topic, we suggest checking out our Combo package with the given link https://www.merchantnavydecoded.com/courses/c/ . It’s a great way to dive deeper into the subject through video explanations. This package covers all the important details and presents them in an easy-to-understand format. Watching the videos will help you grasp the topic better and make learning more enjoyable. So, we highly recommend giving our Combo package a try to enhance your knowledge on the subject.
Disclaimer :- The opinions expressed in this article belong solely to the author and may not necessarily reflect those of Merchant Navy Decoded. We cannot guarantee the accuracy of the information provided and disclaim any responsibility for it. Data and visuals used are sourced from publicly available information and may not be authenticated by any regulatory body. Reviews and comments appearing on our blogs represent the opinions of individuals and do not necessarily reflect the views of Merchant Navy Decoded. We are not responsible for any loss or damage resulting from reliance on these reviews or comments.
Reproduction, copying, sharing, or use of the article or images in any form is strictly prohibited without prior permission from both the author and Merchant Navy Decoded.


[…] The Carnot’s cycle efficiency can be improved by increasing the temperature of the heat source or decreasing the temperature of the cold reservoir. However, the efficiency cannot exceed the Carnot efficiency limit, and any heat engine that violates this limit would violate the second law of thermodynamics. […]