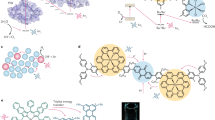
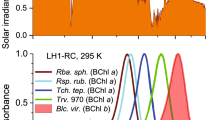
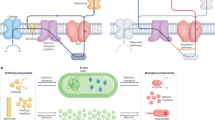
Abstract
Natural photosynthesis is an efficient biochemical process which converts solar energy into energy-rich carbohydrates. By understanding the key photoelectrochemical processes and mechanisms that underpin natural photosynthesis, advanced solar utilization technologies have been developed that may be used to provide sustainable energy to help address climate change. The processes of light harvesting, catalysis and energy storage in natural photosynthesis have inspired photovoltaics, photoelectrocatalysis and photo-rechargeable battery technologies. In this Review, we describe how advanced solar utilization technologies have drawn inspiration from natural photosynthesis, to find sustainable solutions to the challenges faced by modern society. We summarize the uses of advanced solar utilization technologies, such as converting solar energy to electrical and chemical energy, electrochemical storage and conversion, and associated thermal tandem technologies. Both the foundational mechanisms and typical materials and devices are reported. Finally, potential future solar utilization technologies are presented that may mimic, and even outperform, natural photosynthesis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
europa. Climate change policies. European Environment Agency https://www.eea.europa.eu/themes/climate/policy-context (2020).
Hoegh-Guldberg, O. et al. The human imperative of stabilizing global climate change at 1.5 °C. Science 365, 1263 (2019).
Dobson, A., Rowe, Z., Berger, J., Wholey, P. & Caro, T. Biodiversity loss due to more than climate change. Science 374, 698–699 (2021).
Lewis, N. S. Research opportunities to advance solar energy utilization. Science 351, aad1920 (2016). This work presents an insightful review of developments and research opportunities for advance solar energy utilization technologies.
Wang, Q., Pornrungroj, C., Linley, S. & Reisner, E. Strategies to improve light utilization in solar fuel synthesis. Nat. Energy 7, 13–24 (2022).
Croce, R. & van Amerongen, H. Light harvesting in oxygenic photosynthesis: structural biology meets spectroscopy. Science 369, 933 (2020).
Su, X. et al. Structure and assembly mechanism of plant C2S2M2-type PSII–LHCII supercomplex. Science 357, 815–820 (2017).
Qin, X., Suga, M., Kuang, T. & Shen, J.-R. Structural basis for energy transfer pathways in the plant PSI–LHCI supercomplex. Science 348, 989–995 (2015).
Duffy, C. D. P. The simplicity of robust light harvesting. Science 368, 1427–1428 (2020).
Richardson, K. H. et al. Functional basis of electron transport within photosynthetic complex I. Nat. Commun. 12, 5387 (2021).
Schuller, J. M. et al. Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science 363, 257–260 (2019).
Gisriel, C. et al. Structure of a symmetric photosynthetic reaction center-photosystem. Science 357, 1021–1025 (2017).
Suga, M. et al. An oxyl/oxo mechanism for oxygen-oxygen coupling in PSII revealed by an X-ray free-electron laser. Science 366, 334–338 (2019).
Britt, R. D. & Marchiori, D. A. Photosystem II, poised for O2 formation. Science 366, 305–306 (2019).
Hahn, A., Vonck, J., Mills, D. J., Meier, T. & Kuehlbrandt, W. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 360, 620 (2018).
Kern, J. et al. Simultaneous femtosecond X-ray spectroscopy and diffraction of photosystem II at room temperature. Science 340, 491–495 (2013).
Zhang, J. Z. & Reisner, E. Advancing photosystem II photoelectrochemistry for semi-artificial photosynthesis. Nat. Rev. Chem. 4, 6–21 (2020). This review summarizes advances in electrode design and biomaterial interface for semi-artificial photosynthesis through PSII photoelectrochemistry knowledge.
Proppe, A. H. et al. Bioinspiration in light harvesting and catalysis. Nat. Rev. Mater. 5, 828–846 (2020). This review provides comprehensive insights into bioinspiration in light harvesting and catalysis of natural photosynthesis, mainly focusing on the molecules and material design.
Dmytrenko, O., Kunikowska, A., Utter, D., Stewart, F. & Cavanaugh, C. A novel energy-efficient version of the Calvin cycle. N. Biotechnol. 44, S28–S29 (2018).
Schappi, R. et al. Drop-in fuels from sunlight and air. Nature 601, 63–68 (2022).
Ghausi, M. A. et al. CO2 overall splitting by a bifunctional metal-free electrocatalyst. Angew. Chem. Int. Ed. 57, 13135–13139 (2018).
Xie, J., Huang, Y., Wu, M. & Wang, Y. Electrochemical carbon dioxide splitting. ChemElectroChem 6, 1587–1604 (2019).
Maugh, T. H. A new light on photosynthesis. Science 213, 994–996 (1981).
Nocera, D. G. The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012).
Geisz, J. F. et al. Six-junction III–V solar cells with 47.1% conversion efficiency under 143 Suns concentration. Nat. Energy 5, 326–335 (2020).
National Renewable Energy Laboratory (NREL). Best research-cell efficiency chart. NREL https://www.nrel.gov/pv/cell-efficiency.html (2022).
Senthil, R. & Yuvaraj, S. A comprehensive review on bioinspired solar photovoltaic cells. Int. J. Energy Res. 43, 1068–1081 (2019).
Oregan, B. & Gratzel, M. A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991). This article reports the first example of DSSCs.
Hug, H., Bader, M., Mair, P. & Glatzel, T. Biophotovoltaics: natural pigments in dye-sensitized solar cells. Appl. Energy 115, 216–225 (2014).
McDermott, G. et al. Crystal-structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374, 517–521 (1995).
Panda, M. K., Ladomenou, K. & Coutsolelos, A. G. Porphyrins in bio-inspired transformations: light-harvesting to solar cell. Coord. Chem. Rev. 256, 2601–2627 (2012).
Urbani, M., Graetzel, M., Nazeeruddin, M. K. & Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 114, 12330–12396 (2014).
Li, L.-L. & Diau, E. W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 42, 291–304 (2013).
Mathew, S. et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6, 242–247 (2014).
Zeng, K. et al. Molecular engineering strategies for fabricating efficient porphyrin-based dye-sensitized solar cells. Energy Environ. Sci. 13, 1617–1657 (2020).
Park, N.-G. Perovskite solar cells: an emerging photovoltaic technology. Mater. Today 18, 65–72 (2015).
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Luo, D., Su, R., Zhang, W., Gong, Q. & Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 5, 44–60 (2020).
Zhou, Y., Poli, I., Meggiolaro, D., De Angelis, F. & Petrozza, A. Defect activity in metal halide perovskites with wide and narrow bandgap. Nat. Rev. Mater. 6, 986–1002 (2021).
Rombach, F. M., Haque, S. A. & Macdonald, T. J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 14, 5161–5190 (2021).
Jung, E. H. et al. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 567, 511–515 (2019).
Li, M. et al. Dopant-free zinc chlorophyll aggregates as an efficient biocompatible hole transporter for perovskite solar cells. ChemSusChem 9, 2862–2869 (2016).
Kim, G.-W. et al. Hole transport materials in conventional structural (n–i–p) perovskite solar cells: from past to the future. Adv. Energy Mater. 10, 1903403 (2020).
Vohra, V. Natural dyes and their derivatives integrated into organic solar cells. Materials 11, 2579 (2018).
Yao, K. et al. Nano-bio hybrids of plasmonic metals/photosynthetic proteins for broad-band light absorption enhancement in organic solar cells. J. Mater. Chem. A 4, 13400–13406 (2016).
Battaglia, C., Cuevas, A. & De Wolf, S. High-efficiency crystalline silicon solar cells: status and perspectives. Energy Environ. Sci. 9, 1552–1576 (2016).
Yamaguchi, M. et al. GaAs solar cells grown on Si substrates for space use. Prog. Photovolt. 9, 191–201 (2001).
Chapin, D. M., Fuller, C. S. & Pearson, G. L. A new silicon p–n junction photocell for converting solar radiation into electrical power. J. Appl. Phys. 25, 676–677 (1954).
Shockley, W. & Queisser, H. J. Detailed balance limit of efficiency of p–n junction solar cells. J. Appl. Phys. 32, 510–519 (1961).
Wrobel, D. From natural photosynthesis to molecular photovoltaics. Mol. Cryst. Liq. Cryst. 627, 4–22 (2016).
Godin, R. & Durrant, J. R. Dynamics of photoconversion processes: the energetic cost of lifetime gain in photosynthetic and photovoltaic systems. Chem. Soc. Rev. 50, 13372–13409 (2021).
Berardi, S. et al. Molecular artificial photosynthesis. Chem. Soc. Rev. 43, 7501–7519 (2014).
Whang, D. R. & Apaydin, D. H. Artificial photosynthesis: learning from nature. ChemPhotoChem 2, 148–160 (2018).
Wang, Q. & Domen, K. Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies. Chem. Rev. 120, 919–985 (2020).
Kim, J. H., Hansora, D., Sharma, P., Jang, J.-W. & Lee, J. S. Toward practical solar hydrogen production — an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Yoshino, S., Takayama, T., Yamaguchi, Y., Iwase, A. & Kudo, A. CO2 reduction using water as an electron donor over heterogeneous photocatalysts aiming at artificial photosynthesis. Acc. Chem. Res. 55, 966–977 (2022).
Nasir, J. A. et al. Photocatalytic Z-scheme overall water splitting: recent advances in theory and experiments. Adv. Mater. 33, 2105195 (2021).
Jiang, C., Moniz, S. J. A., Wang, A., Zhang, T. & Tang, J. Photoelectrochemical devices for solar water splitting—materials and challenges. Chem. Soc. Rev. 46, 4645–4660 (2017).
Liu, J. et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347, 970–974 (2015).
Song, H., Luo, S. Q., Huang, H. M., Deng, B. W. & Ye, J. H. Solar-driven hydrogen production: recent advances, challenges, and future perspectives. ACS Energy Lett. 7, 1043–1065 (2022).
Zhang, C. X. et al. A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis. Science 348, 690–693 (2015).
Suga, M. et al. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 543, 131–135 (2017).
Barber, J. A mechanism for water splitting and oxygen production in photosynthesis. Nat. Plants 3, 17041 (2017).
Barber, J. Photosynthetic water splitting provides a blueprint for artificial leaf technology. Joule 1, 5–9 (2017).
Zhang, B. & Sun, L. Across the board: Licheng Sun on the mechanism of O−O bond formation in photosystem II. ChemSusChem 12, 3401–3404 (2019).
Gersten, S. W., Samuels, G. J. & Meyer, T. J. Catalytic oxidation of water by an oxo-bridged ruthenium dimer. J. Am. Chem. Soc. 104, 4029–4030 (1982).
Yi, J. et al. Electrostatic interactions accelerating water oxidation catalysis via intercatalyst O–O coupling. J. Am. Chem. Soc. 143, 2484–2490 (2021).
Okamura, M. et al. A pentanuclear iron catalyst designed for water oxidation. Nature 530, 465–468 (2016).
Nguyen, A. I. et al. Mechanistic investigations of water oxidation by a molecular cobalt oxide analogue: evidence for a highly oxidized intermediate and exclusive terminal oxo participation. J. Am. Chem. Soc. 137, 12865–12872 (2015).
Wang, Y. et al. Mimicking natural photosynthesis: solar to renewable H2 fuel synthesis by Z-scheme water splitting systems. Chem. Rev. 118, 5201–5241 (2018).
Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 3, 1486–1503 (2013).
Zhao, D. et al. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 6, 388–397 (2021).
Qiu, B. et al. Integration of redox cocatalysts for artificial photosynthesis. Energy Environ. Sci. 14, 5260–5288 (2021).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2, 509–520 (2018).
Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100 m2 scale. Nature 598, 304–307 (2021).
Zhao, Y. et al. Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks. Chem. Soc. Rev. 48, 1972–2010 (2019).
Roy, S. C., Varghese, O. K., Paulose, M. & Grimes, C. A. Toward solar fuels: photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 4, 1259–1278 (2010).
Wang, Y. et al. Direct and indirect Z-scheme heterostructure-coupled photosystem enabling cooperation of CO2 reduction and H2O oxidation. Nat. Commun. 11, 1–11 (2020).
Wang, Q. et al. Molecularly engineered photocatalyst sheet for scalable solar formate production from carbon dioxide and water. Nat. Energy 5, 703–710 (2020).
Jiang, Z. et al. Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction. Nature 586, 549–554 (2020). This article reports a MOF material for photocatalytic CO2 reduction and water oxidation.
Schmidt, D., Hager, M. D. & Schubert, U. S. Photo-rechargeable electric energy storage systems. Adv. Energy Mater. 6, 1500369 (2016).
Johnson, M. P. Understanding biochemistry 2. Essays in Biochemistry 60, 255–273 (2016).
Fu, Y. et al. Integrated power fiber for energy conversion and storage. Energy Environ. Sci. 6, 805–812 (2013).
Tian, Z. et al. Ultrafast rechargeable Zn micro-batteries endowing a wearable solar charging system with high overall efficiency. Energy Environ. Sci. 14, 1602–1611 (2021).
Chen, P., Li, G.-R., Li, T.-T. & Gao, X.-P. Solar-driven rechargeable lithium–sulfur battery. Adv. Sci. 6, 1900620 (2019).
Takshi, A., Aljafari, B., Kareri, T. & Stefanakos, E. A critical review on the voltage requirement in hybrid cells with solar energy harvesting and energy storage capability. Batteries Supercaps 4, 252–267 (2021).
Gibson, T. L. & Kelly, N. A. Solar photovoltaic charging of lithium-ion batteries. J. Power Sources 195, 3928–3932 (2010).
Um, H.-D. et al. Monolithically integrated, photo-rechargeable portable power sources based on miniaturized Si solar cells and printed solid-state lithium-ion batteries. Energy Environ. Sci. 10, 931–940 (2017).
Xu, J., Chen, Y. & Dai, L. Efficiently photo-charging lithium-ion battery by perovskite solar cell. Nat. Commun. 6, 1–7 (2015).
Gurung, A. et al. Highly efficient perovskite solar cell photocharging of lithium ion battery using DC–DC booster. Adv. Energy Mater. 7, 1602105 (2017).
Du, P. et al. Self-powered electronics by integration of flexible solid-state graphene-based supercapacitors with high performance perovskite hybrid solar cells. Adv. Funct. Mater. 25, 2420–2427 (2015).
Li, C. et al. Flexible perovskite solar cell-driven photo-rechargeable lithium-ion capacitor for self-powered wearable strain sensors. Nano Energy 60, 247–256 (2019).
Gurung, A. & Qiao, Q. Solar charging batteries: advances, challenges, and opportunities. Joule 2, 1217–1230 (2018).
Lv, J., Xie, J., Mohamed, A. G. A., Zhang, X. & Wang, Y. Photoelectrochemical energy storage materials: design principles and functional devices towards direct solar to electrochemical energy storage. Chem. Soc. Rev. 51, 1511–1528 (2022). This review summarizes the design principles of photoelectrochemical energy storage materials for direct solar to electrochemical energy storage.
Paolella, A. et al. Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat. Commun. 8, 14643 (2017).
Lv, J. Q. et al. Direct solar-to-electrochemical energy storage in a functionalized covalent organic framework. Angew. Chem. Int. Ed. 57, 12716–12720 (2018).
Boruah, B. D., Wen, B. & De Volder, M. Light rechargeable lithium-ion batteries using V2O5 cathodes. Nano Lett. 21, 3527–3532 (2021).
Deka Boruah, B. et al. Vanadium dioxide cathodes for high-rate photo-rechargeable zinc-ion batteries. Adv. Energy Mater. 11, 2100115 (2021).
Boruah, B. D. et al. Photo-rechargeable zinc-ion batteries. Energy Environ. Sci. 13, 2414–2421 (2020).
Huang, Y. et al. Atomic modulation and structure design of carbons for bifunctional electrocatalysis in metal–air batteries. Adv. Mater. 31, 1803800 (2019).
Lv, J. et al. A photo-responsive bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nano Energy 43, 130–137 (2018).
Jia, C. et al. Ultra-large sized siloxene nanosheets as bifunctional photocatalyst for a Li–O2 battery with superior round-trip efficiency and extra-long durability. Angew. Chem. Int. Ed. 60, 11257–11261 (2021).
Lv, Q. et al. Semiconducting metal-organic polymer nanosheets for a photoinvolved Li–O2 battery under visible light. J. Am. Chem. Soc. 143, 1941–1947 (2021).
Qiao, G.-Y. et al. Perovskite quantum dots encapsulated in a mesoporous metal–organic framework as synergistic photocathode materials. J. Am. Chem. Soc. 143, 14253–14260 (2021).
Fukuzumi, S. Development of bioinspired artificial photosynthetic systems. Phys. Chem. Chem. Phys. 10, 2283–2297 (2008).
Li, D., Shi, J. & Li, C. Transition-metal-based electrocatalysts as cocatalysts for photoelectrochemical water splitting: a mini review. Small 14, 1704179 (2018).
Hemmerling, J. R., Mathur, A. & Linic, S. Design principles for efficient and stable water splitting photoelectrocatalysts. Acc. Chem. Res. 54, 1992–2002 (2021).
Jia, J. et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 7, 1–6 (2016).
Wang, J. et al. Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 49, 9154–9196 (2020).
Yu, Z.-Y. et al. Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects. Adv. Mater. 33, 2007100 (2021).
Deng, C. et al. Earth-abundant metal-based electrocatalysts promoted anodic reaction in hybrid water electrolysis for efficient hydrogen production: recent progress and perspectives. Adv. Energy Mater. 12, 2201047 (2022).
Lei, L. et al. Demystifying the active roles of NiFe-based oxides/(oxy)hydroxides for electrochemical water splitting under alkaline conditions. Coord. Chem. Rev. 408, 213177 (2020).
Roger, I., Shipman, M. A. & Symes, M. D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 1, 0003 (2017).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Yu, Z., Li, F. & Sun, L. Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components. Energy Environ. Sci. 8, 760–775 (2015).
Xiao, Y. et al. Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water oxidation. Nat. Catal. 3, 932–940 (2020).
Pan, L. et al. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat. Commun. 11, 1–10 (2020).
Wang, H. et al. Highly active deficient ternary sulfide photoanode for photoelectrochemical water splitting. Nat. Commun. 11, 1–11 (2020).
Thalluri, S. M. et al. Strategies for semiconductor/electrocatalyst coupling toward solar-driven water splitting. Adv. Sci. 7, 1902102 (2020).
Zhong, S. et al. Hybrid cocatalysts in semiconductor-based photocatalysis and photoelectrocatalysis. J. Mater. Chem. A 8, 14863–14894 (2020).
Polman, A., Knight, M., Garnett, E. C., Ehrler, B. & Sinke, W. C. Photovoltaic materials: present efficiencies and future challenges. Science 352, aad4424 (2016).
Cheng, W.-H. et al. Monolithic photoelectrochemical device for direct water splitting with 19% efficiency. ACS Energy Lett. 3, 1795–1800 (2018).
Schneidewind, J. How much technological progress is needed to make solar hydrogen cost-competitive? Adv. Energy Mater. 12, 2200342 (2022).
Feng, J. et al. Non-oxide semiconductors for artificial photosynthesis: progress on photoelectrochemical water splitting and carbon dioxide reduction. Nano Today 30, 100830 (2020).
Tan, J. et al. Hydrogel protection strategy to stabilize water-splitting photoelectrodes. Nat. Energy 7, 537–547 (2022).
Zhang, M., Xuan, X., Wang, W., Ma, C. & Lin, Z. Anode photovoltage compensation-enabled synergistic CO2 photoelectrocatalytic reduction on a flower-like graphene-decorated Cu foam cathode. Adv. Funct. Mater. 30, 2005983 (2020).
Foster, B. M. et al. Catalytic mismatching of CuInSe2 and Ni3Al demonstrates selective photoelectrochemical CO2 reduction to methanol. ACS Appl. Energy Mater. 3, 109–113 (2020).
Ding, P., Jiang, T., Han, N. & Li, Y. Photocathode engineering for efficient photoelectrochemical CO2 reduction. Mater. Today Nano 10, 100077 (2020).
Chu, S. et al. Decoupling strategy for enhanced syngas generation from photoelectrochemical CO2 reduction. iScience 23, 101390 (2020).
Andrei, V., Reuillard, B. & Reisner, E. Bias-free solar syngas production by integrating a molecular cobalt catalyst with perovskite–BiVO4 tandems. Nat. Mater. 19, 189–194 (2020).
Andrei, V. et al. Floating perovskite–BiVO4 devices for scalable solar fuel production. Nature 608, 518–522 (2022).
Wang, X. Y. et al. Rechargeable Zn–CO2 electrochemical cells mimicking two-step photosynthesis. Adv. Mater. 31, e1807807 (2019). This article reports a decoupled semi-artificial photosynthesis.
Xie, J. F. et al. Reversible aqueous zinc–CO2 batteries based on CO2–HCOOH interconversion. Angew. Chem. Int. Ed. 57, 16996–17001 (2018).
Xie, J. & Wang, Y. Recent development of CO2 electrochemistry from Li–CO2 batteries to Zn–CO2 batteries. Acc. Chem. Res. 52, 1721–1729 (2019).
Xie, J. F., Zhou, Z. & Wang, Y. B. Metal–CO2 batteries at the crossroad to practical energy storage and CO2 recycle. Adv. Funct. Mater. 30, 1908285 (2020).
Wang, X. Y. et al. A photovoltaic-driven solid-state Zn–CO2 electrochemical cell system with sunlight-insusceptible chemical production. J. Mater. Chem. A 8, 13806–13811 (2020). This article reports a decoupled artificial leaf device.
Wang, X.-X. et al. A renewable light-promoted flexible Li–CO2 battery with ultrahigh energy efficiency of 97.9%. Small 17, 2100642 (2021).
Guan, D.-H. et al. Light/electricity energy conversion and storage for a hierarchical porous In2S3@CNT/SS cathode towards a flexible Li–CO2 battery. Angew. Chem. Int. Ed. 59, 19518–19524 (2020).
Li, J. X. et al. High-efficiency and stable Li–CO2 battery enabled by carbon nanotube/carbon nitride heterostructured photocathode. Angew. Chem. Int. Ed. 61, e202114612 (2022).
Liu, X. et al. Ultrathin p–n type Cu2O/CuCoCr-layered double hydroxide heterojunction nanosheets for photo-assisted aqueous Zn–CO2 batteries. J. Mater. Chem. A 9, 26061–26068 (2021).
Krishna, A. et al. Infrared optical and thermal properties of microstructures in butterfly wings. Proc. Natl Acad. Sci. USA 117, 1566–1572 (2020).
Weinstein, L. A. et al. Concentrating solar power. Chem. Rev. 115, 12797–12838 (2015).
Zhang, C., Liang, H.-Q., Xu, Z.-K. & Wang, Z. Harnessing solar-driven photothermal effect toward the water–energy nexus. Adv. Sci. 6, 1900883 (2019).
Wang, L., Feng, Y., Wang, K. & Liu, G. Solar water sterilization enabled by photothermal nanomaterials. Nano Energy 87, 106158 (2021).
Bai, Y., Jantunen, H. & Juuti, J. Energy harvesting research: the road from single source to multisource. Adv. Mater. 30, 1707271 (2018).
Zhang, Y., Wu, K. & Fu, Q. A structured phase change material with controllable thermoconductive highways enables unparalleled electricity via solar-thermal-electric conversion. Adv. Funct. Mater. 32, 2109255 (2022).
Yang, L., Chen, Z.-G., Dargusch, M. S. & Zou, J. High performance thermoelectric materials: progress and their applications. Adv. Energy Mater. 8, 1701797 (2018).
Wang, Y. et al. Efficient photo-thermo-electric conversion using polyoxovanadate in ionic liquid for low-grade heat utilization. ChemSusChem 14, 5434–5441 (2021).
Bayrak, F., Abu-Hamdeh, N., Alnefaie, K. A. & Oztop, H. F. A review on exergy analysis of solar electricity production. Renew. Sustain. Energy Rev. 74, 755–770 (2017).
Zhang, Y., Umair, M. M., Zhang, S. & Tang, B. Phase change materials for electron-triggered energy conversion and storage: a review. J. Mater. Chem. A 7, 22218–22228 (2019).
Li, T. et al. Highly conductive phase change composites enabled by vertically-aligned reticulated graphite nanoplatelets for high-temperature solar photo/electro-thermal energy conversion, harvesting and storage. Nano Energy 89, 106338 (2021).
Zhang, W., Ling, Z., Fang, X. & Zhang, Z. Anisotropically conductive Mg(NO3)2·6H2O/g-C3N4-graphite sheet phase change material for enhanced photo-thermal storage. Chem. Eng. J. 430, 132997 (2022).
Liu, H. et al. Photothermal catalysts for hydrogenation reactions. Chem. Commun. 57, 1279–1294 (2021).
Romero, M. & Steinfeld, A. Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 5, 9234–9245 (2012).
Chueh, W. C. et al. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330, 1797–1801 (2010).
Zhou, L. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69–72 (2018).
Rej, S. et al. Determining plasmonic hot electrons and photothermal effects during H2 evolution with TiN–Pt nanohybrids. ACS Catal. 10, 5261–5271 (2020).
Cai, M. et al. Greenhouse-inspired supra-photothermal CO2 catalysis. Nat. Energy 6, 807–814 (2021).
Fang, S. & Hu, Y. H. Thermo-photo catalysis: a whole greater than the sum of its parts. Chem. Soc. Rev. 51, 3609–3647 (2022).
Tao, P. et al. Solar-driven interfacial evaporation. Nat. Energy 3, 1031–1041 (2018).
Jain, P. K., Huang, X., El-Sayed, I. H. & El-Sayed, M. A. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 41, 1578–1586 (2008).
Chen, X. et al. Scaling up nanoscale water-driven energy conversion into evaporation-driven engines and generators. Nat. Commun. 6, 1–7 (2015).
Yang, P. et al. Solar-driven simultaneous steam production and electricity generation from salinity. Energy Environ. Sci. 10, 1923–1927 (2017).
Chang, C., Wang, Z., Fu, B. & Ji, Y. High-efficiency solar thermoelectric conversion enabled by movable charging of molten salts. Sci. Rep. 10, 20500 (2020).
Mehrali, M., ten Elshof, J. E., Shahi, M. & Mahmoudi, A. Simultaneous solar-thermal energy harvesting and storage via shape stabilized salt hydrate phase change material. Chem. Eng. J. 405, 126624 (2021).
Marxer, D., Furler, P., Takacs, M. & Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 10, 1142–1149 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22105037, 22279141, 22205238, 21872147 and 22022110), Key Research Program of Frontier Sciences, CAS (No. ZDBS-LY-SLH028), National key Research & Development Program of China (2021YFA1501500 and 2021YFA1202700), CAS Key Laboratory of Urban Pollutant Conversion Joint Research Fund (KLUPC-2020-3), Youth Innovation Promotion Association CAS (2022308) to J.X., Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (No. 2021ZZ106), Science and Technology Service Network Initiative (KFJ-STS-QYZD-2021-09-23) and Natural Science Foundation of Fujian Province (No. 2020J01934).
Author information
Authors and Affiliations
Contributions
J.L., J.X. and A.G.A.M. contributed equally to this work. J.L., J.X., A.G.A.M. and Y.W. wrote this Review together. X.Z., Y.F., L.J., D.Y. and E.Z. helped draw the figures. Y.W. is in charge of all the manuscript. All authors contributed to the discussion and writing of this Review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, J., Xie, J., Mohamed, A.G.A. et al. Solar utilization beyond photosynthesis. Nat Rev Chem 7, 91–105 (2023). https://doi.org/10.1038/s41570-022-00448-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-022-00448-9
This article is cited by
-
Nature-inspired interfacial engineering for energy harvesting
Nature Reviews Electrical Engineering (2024)
-
Azobenzene-based ultrathin peptoid nanoribbons for the potential on highly efficient artificial light-harvesting
Science China Chemistry (2024)
-
A leap towards solar-powered water and energy solutions
Nature Water (2023)
-
The future is light
Nature Reviews Chemistry (2023)
-
Improving Wheat Growth and Nutrient Uptake in Calcareous Soil: a Novel Approach with Carbon Dots as a Slow-Release Zinc Fertilizer
Journal of Soil Science and Plant Nutrition (2023)