For decades public health officials thought the Zika virus caused only relatively mild illnesses in people. Since the start of an outbreak in Brazil in 2015, however, it has become horribly clear that the virus can pass from pregnant women to their fetuses, with devastating consequences. The virus kills some of the unborn children and leaves others with severe brain damage, including smaller than normal heads (a condition called microcephaly). How the virus reaches the fetus is a mystery because to get there, it must cross the placenta, a pancake-shaped organ that connects the developing infant to the mother and that manages to block transmission of other closely related mosquito-borne viruses, such as dengue and yellow fever, from mother to baby.
In the past several years this puzzle and others have drawn new research attention to the placenta, which is the first and largest organ to develop after conception. It is a product of the embryo, not the mother, and among other things, it supplies the fetus with nutrients and oxygen and disposes of waste products. Yet despite its critical role in pregnancy, it is arguably the least understood organ in the human body.
The placenta's vulnerability to Zika is not the only conundrum. Researchers have long wondered why a mother's immune system does not recognize the placenta and the fetus as genetically foreign and therefore target them for attack. In fact, not only does the mother's immune system keep itself in check, it actually aids in the proper development and functioning of the placenta.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Research carried out by our laboratories and others has begun to yield fascinating insights into these questions. Through these findings, we are increasingly realizing that certain complications of pregnancy—once thought to be caused entirely by problems in the mother's body—are attributable to defects in the placenta or its interaction with the uterus. What is more, subtle variations in the placenta might affect a person's health later in life.
Rapid Development
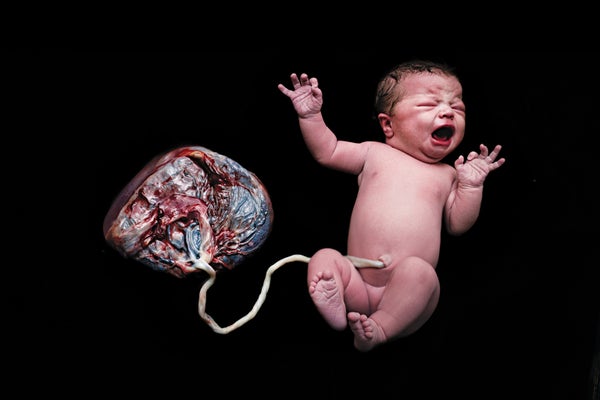
Although mysteries related to the placenta abound, two aspects are well understood: the organ's structure and the basic steps of its development. At the time of delivery, the slablike placenta weighs just more than a pound and has two distinct sides: the part attached to the mother's uterine wall before delivery, which has the appearance of a blood-soaked sponge, and the one facing the baby, which contains an array of blood vessels that traverse the umbilical cord [see illustration below].

This side of the placenta, shown after birth with the umbilical cord, faced the baby during pregnancy. The flip side, which resembles a blood-filled sponge, was attached to the mother's uterine wall. Credit: Norm Barker
The placenta develops quickly because it has to do the jobs of other developing organs until they become fully functional—like the liver, it metabolizes nutrients; like the lungs, it exchanges oxygen for carbon dioxide; like the kidneys, it excretes wastes. Less than a week after a sperm fertilizes an egg, specialized cells called trophoblasts emerge on the surface of the embryo. The first task for these cells (which also produce hormones that alert the mother's body to the presence of the embryo) is to burrow into the uterine wall. There the trophoblasts divide rapidly, forming projections that radiate into the uterus. One layer is composed of cells known as cytotrophoblasts. Another layer of fused cells (known as syncytiotrophoblasts) becomes the surface of the placenta. Eventually the placenta takes the form of a disk that is attached to the uterine wall by branching structures.
During the second and third weeks after fertilization, these branches begin to fill with support cells and blood vessels. By around the time a woman learns she is pregnant, the mature configuration of these structures, now known as chorionic villi, has been established.
In the placenta's race with time to become fully functional early in pregnancy, its ability to reroute maternal blood flow to itself is paramount. The extraordinary journey that cytotrophoblasts take makes this feat possible. First, the cells attach themselves to the surface of the uterine wall, then they migrate ever deeper inside. Nearly two decades ago one of us (Fisher) discovered that the cytotrophoblasts transform themselves during this process so that they mimic the cells that typically line the blood vessels. This mimicry enables the cytotrophoblasts to breach the mother's oxygen-laden arteries [see graphic below]. Once inside, they climb up each vessel's inner lining, replacing it as they go.
Because of the cells' machinations, the mother's arteries in the uterus expand and lose their normal “tone,” which would otherwise restrict the amount of blood they can carry. By the end of the first trimester, the arteries open into the spaces between the chorionic villi, delivering the large quantities of maternal blood (and thus nutrients and oxygen) required for the offspring's growth. The cytotrophoblasts also invade the uterine veins, enabling blood to flow from the placenta back to the mother's body, which completes the circuit and carries carbon dioxide and other waste products away from the fetus.
.png?w=900)
Credit: AXS Biomedical Studio; Courtesy of Susan J. Fisher (micrograph)
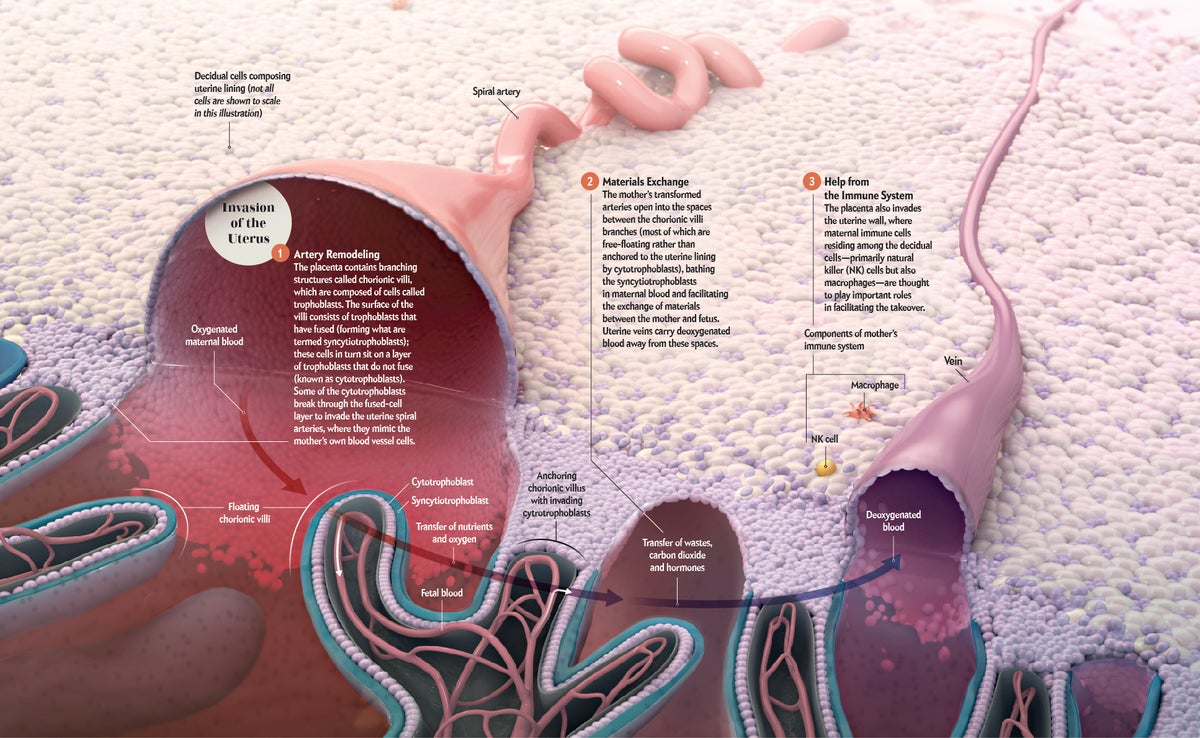
Credit: AXS Biomedical Studio; Courtesy of Susan J. Fisher (micrograph)
Blood from the mother's arteries bathes the surface of the placenta just a few cell layers away from the offspring's own blood vessels. This proximity maximizes the exchange of nutrients, gases and waste products. Researchers have also determined in the past few years that the placenta releases large quantities of the offspring's DNA into maternal blood, which makes it possible to do prenatal genetic testing with only a blood sample from the mother. Such tests are rapidly replacing older, more invasive procedures, such as chorionic villus sampling and amniocentesis.
Environmental Influences
The genes of the fetus direct much of the placenta's development, but the microenvironment surrounding the organ also plays a vital role. Research over the past two decades has begun to reveal how much a successful pregnancy relies on the interchange between maternal cells in the uterine tissue and the invading branches of the placenta. The areas where the placenta and uterus meet—the so-called maternal-fetal interface—harbor various kinds of immune cells called leukocytes that migrate to the site from the mother's blood. The trophoblasts from the fetus maintain an ongoing dialogue with these leukocytes and other uterine cells to keep the placenta working properly.
The behavior of the maternal immune cells is surprising. The placenta, which derives half of its genes from the father, is inherently foreign to the mother. This foreignness raises the question of how the placenta escapes rejection by immunological processes that would otherwise recognize and destroy such an invader, as in the case of a conventional organ transplant. Investigators now know that changes in a mother's immune system help her “tolerate” the placenta. Local processes that operate within the uterus also play a part. For example, research on mice published in 2012 by one of us (Erlebacher) showed that the leukocytes that usually reject organ transplants are unable to accumulate in the uterine wall near the invading placenta.
The mother's body does not simply tolerate the placenta, however. It actively promotes the invasive growth of fetal tissues. Starting in the 1980s, for example, researchers discovered that a type of leukocyte called a natural killer cell is abundant on the uterine side of the maternal-fetal interface. As a general rule, these specialized cells kill tumors and virus-infected cells. But in the 1990s investigators led by B. Anne Croy, now at Queen's University in Ontario, made the counterintuitive discovery that natural killer cells also support placental development—in particular, the remodeling of uterine arteries by cytotrophoblasts. Presumably uterine natural killer cells produce substances that promote the loss of the original maternal cells that line the arteries, thus facilitating the subsequent takeover of these vessels by placental cells.
Problems at the Interface
Given the breakneck pace of placental development and the many cell types that make up the placenta and the uterine wall, it should perhaps come as no surprise that errors can arise during the formation of the maternal-fetal interface. Such mistakes can cause a diverse array of pregnancy complications, the most important of which are termed the “great obstetrical syndromes.” They include preterm birth (delivery occurs before 37 weeks of pregnancy), intrauterine growth restriction (the baby is smaller than expected), and preeclampsia (the mother suddenly develops high blood pressure and vascular damage).
Recent studies of the placenta have helped elucidate the origins of some of these conditions. Preeclampsia, for example, was once known as toxemia: physicians believed that it occurred because the placenta released toxins into the mother's blood. Although the precise mechanisms that cause preeclampsia—which affects about 8 percent of first-time pregnancies—are still unknown, investigators have found that it is associated with distinct structural deformities in the maternal-fetal interface. Experts now believe that the condition stems from an insufficient cytotrophoblast invasion of the uterine arteries during the first half of pregnancy. The inadequate blood supply restricts fetal growth. Eventually the abnormally developed placenta does release substances that are toxic to the mother, particularly to her circulatory system, but these toxins do not appear to be the root cause, as was previously thought. Rather they are likely a consequence of the disease. If left untreated, preeclampsia can lead to serious or even fatal damage to both mother and child.

Five-week-old placenta already has a branching structure but is relatively pale because it is not yet perfused by the mother's blood. This one is from an aborted pregnancy; normally at this stage it would entirely surround the embryo and its amniotic sac. Credit: Joo Lee Getty Images
Exactly why the placenta fails to work properly in cases of preeclampsia remains obscure. The dysfunction may arise within the cytotrophoblasts or the various maternal cells, or some combination thereof. Intriguingly, the ability of natural killer cells to detect foreign tissue may be a contributing factor. Research conducted by Ashley Moffett, a specialist in reproductive immunology at the University of Cambridge, suggests that if the placenta and mother are too similar immunologically, the natural killer cells may not be able to fully support the replacement of maternal cells from the inner lining of the uterine arteries with cells from the placenta.
Another of the great obstetrical syndromes—preterm birth—has recently raised alarm bells because its incidence is increasing worldwide. According to the Centers for Disease Control and Prevention, this disorder now affects about one in 10 pregnancies in the U.S. Although intrauterine infection can trigger early delivery, many preterm births have no clear-cut cause. In fact, scientists still do not understand what triggers a normal birth at the end of pregnancy, one of the major unsolved questions in human biology. Presumably a “clock” counts down the 280 days of human gestation. We know that when the alarm goes off, it initiates an inflammatory cascade within the uterus that is probably the immediate cause of uterine contraction and delivery. But where is the clock ticking? Is it in the fetus, placenta or uterus? It is easy to imagine that defective placental development early in gestation might gum up the inner workings of a birth clock, although this idea remains speculative.
The great obstetrical syndromes overlap in their signs and in their underlying mechanisms. Shallow cytotrophoblast invasion, which is consistently associated with preeclampsia, for example, is also a feature of intrauterine growth restriction and some cases of preterm birth. A better understanding of how problems at the maternal-fetal interface diverge into very different complications might suggest ways to intervene more effectively.
Lasting Imprints
Such major gestational irregularities have obvious harmful effects on newborns, from treatable conditions that require hospitalization in neonatal intensive care units to permanent neurological impairments. The ill effects of an inadequate environment for fetal growth do not end with infancy, however; they may manifest decades later as adult diseases, and there is reason to believe that flaws in placental function play a part here, too.
The idea that conditions in the womb can affect health in later life is termed the fetal origins hypothesis. It was first proposed in the 1980s by the late British epidemiologist David Barker to try to explain the high incidence of cardiovascular disease and diabetes in poor areas of England. Barker noted that adults with these chronic conditions were more likely to have been underweight at birth, possibly reflecting suboptimal nutritional status. Some investigators think that malnutrition and faulty placental function may change the way a baby's genes direct its development during pregnancy—but the mechanics that underlie this process are unknown. Epidemiological evidence also strongly indicates that children born to women who contract certain infections—such as an influenza virus—during pregnancy have an increased risk of neurodevelopmental and psychiatric disorders, including autism, bipolar disorder and schizophrenia.
A mouse study published in 2016 by immunologists Dan R. Littman of New York University and Jun R. Huh of the University of Massachusetts Medical School suggests how a flu infection might subtly change the course of brain development in a way that only becomes evident years or decades later. Previously scientists knew that virus-mimicking agents that cause systemic inflammation in pregnant mice also cause autismlike behaviors in their offspring. Littman, Huh and their colleagues showed that the inflammation-inducing agent is interleukin-17 (IL-17) and that it is produced by the mother's immune cells. Using sophisticated imaging techniques, the team showed that the protein was directly responsible for subtle structural changes in the brains of affected mice.
But how does maternal IL-17 cross the placenta to reach the fetal brain when many other molecules of a similar size cannot get through? One possibility is that, for some reason, the placenta actively transports IL-17 from maternal blood to the fetal circulatory system, enabling it to access the brain. Another intriguing possibility is that some of the mother's cells that are making IL-17 themselves traverse the placenta to reach the fetus.
Crossing Over
The Zika pandemic is a graphic illustration of the damage that can be done when a virus that infects the mother learns how to cross the placenta. At the moment, though, researchers have more questions than answers as to how Zika causes the health problems that have arisen in affected babies.
Given that investigators only recently made the association between Zika virus infections during pregnancy and adverse outcomes, it is not surprising that very little is known about how Zika reaches the fetus. Even the rate of birth defects is unclear and seems to vary by location. In a U.S.-based study published in January 2017 in JAMA, researchers at public health departments and the CDC found birth defects in only 6 percent of the fetuses or infants of mothers with possible Zika infections. A Brazilian study published the previous month in the New England Journal of Medicine, however, suggested that almost half of infected fetuses could have some form of damage. In addition, some Brazilian babies have developed neurological problems after initially being evaluated as normal. Because Zika's most damaging effects—particularly microcephaly—seem to be more prevalent in Brazil than elsewhere, some researchers have speculated that a chemical in the Brazilian environment may weaken the placenta and make it more susceptible to Zika. Alternatively, simultaneous infection with another microbe prevalent in Brazil might be the culprit.
Also in question is how Zika reaches the fetus. Does it plow through the placenta from the maternal side, infecting every cell type in its way, or is it chaperoned by particular cell types—such as maternal immune cells? Alternatively, we know that certain pathogens can ascend from the vagina to the uterus, thereby gaining access to fetal tissues. However the Zika virus reaches the fetal tissues, it establishes a firm foothold once there: molecular pathologists at the CDC have reported that the Zika virus can persist in the placenta for months and can continue to replicate in an infant's brain even after birth.
Zika is not, of course, the only pathogen that can cross the placenta and harm the fetus. Around the globe, an estimated 100,000 babies are born every year with congenital rubella, which can cause deafness, eye abnormalities, heart disease and other serious problems. Malaria, herpes and Ebola can all cause lethal damage during pregnancy. The precise means by which they invade the fetus remain to be determined. But it appears likely that some pathogens are better able to infect the placenta's trophoblasts, particularly in early gestation. Immune defense mechanisms at the maternal-fetal interface are probably also poor gatekeepers at times, given that the uterine lining has two inherently contradictory jobs. On the one hand, it must protect the fetus and placenta from infection. On the other hand, as alluded to earlier, it must prevent maternal immune responses from becoming so robust that the placenta is harmed.
With so much left to learn about the placenta and pregnancy, the Eunice Kennedy Shriver National Institute of Child Health and Human Development three years ago launched the Human Placenta Project, which aims to understand this enigmatic organ that “influences not just the health of a woman and her fetus during pregnancy, but also the lifelong health of both.” Along with efforts to cure HIV, cancer and cardiovascular disease, understanding the placenta should be given the highest priority on the national health research agenda.